9.1 Drugs for Depression
CNS Pharmacology – Antidepressants
In this topic, we will look at the pharmacology of antidepressants. We will explore the signs and symptoms, pathogenesis and drug treatments of depression. The drug classes we will investigate include TCAs, SSRIs, SNRIs and MAOIs. Please note if you or someone you know is struggling with mental health call a crisis line like Lifeline 13 11 14.
Learning Outcomes
By the end of this module, it is expected students should be able to demonstrate and apply knowledge of:
- Nature and symptoms of depression
- Pathogenesis of depression
- Neurochemical theories of depression
- Mechanisms of action of antidepressants
- Adverse effects
- Serotonin syndrome.
Introduction
Depression is a mental health disorder characterised by persistently depressed mood, negative mental status or loss of interest in activities, causing significant impairment in daily life that can lead to suicide. On the other hand, depressed mood is part of the range of normal expression in human in response to emotional stress (e.g., losing of love one). Globally, more than 264 million people of all ages suffer from depression (4.1% population in Australia). One in six people suffers from depression sometime in the life where depression is more prevalent in female (1/5) than male (1/8). Unfortunately, despite its high prevalence, major depression has been underdiagnosed and undertreated.
Depression is the most common affective disorder
Affective disorders (or mood disorders) are either unipolar or bipolar. Unipolar depressive disorders are characterised by single or recurrent major depressive episodes. Bipolar affective disorder is characterised by alternating manic and depressive episodes. Depression is the most common affective disorder and can be classified as either exogenous/reactive or endogenous. Exogenous depression appears to be triggered by a specific event while endogenous depression has no obvious external cause. The symptoms of depression encompass emotional and biological components. Examples of symptoms include:
- Sadness
- Loss of interest
- Anhedonia
- Changes in appetite
- Feelings of guilt
- Low self-esteem or self-worth
- Sleep disturbance
- Feelings of tiredness
- Poor concentration
- Suicidal ideation.
📚 Read/Explore
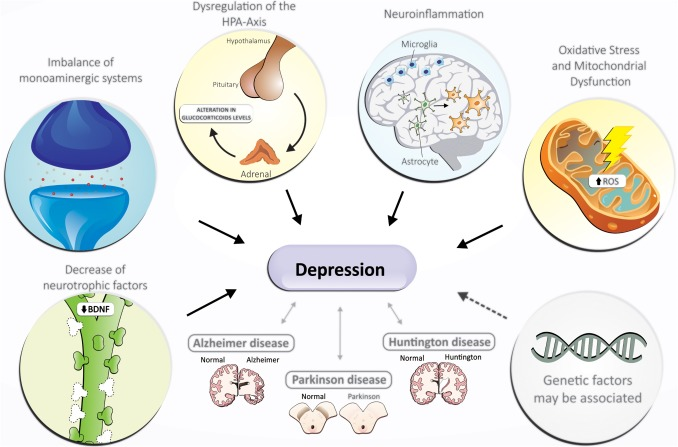
The exact pathogenesis of depression is still unclear
The exact pathogenesis of depression is still unclear. Brain imaging studies have indicated that the prefrontal cortex, amygdala and hippocampus may be involved. Mechanisms of interest include:
- Upregulation of beta1, alpha2 and 5-HT2 receptors.
- Lowered levels of brain-derived neurotrophic factor (BDNF).
- Neurodegeneration.
- Elevated levels of corticotrophin-releasing factor (CRF).
- Reduced amount of monoamine neurotransmitters in neuronal pathways.
Overall, no single theory explains all observations of depression. A combination of genetic, psychosocial and biological factors probably leads to common pathways that result in depression.
📺 Watch the following lecture on the introduction to depression (10 minutes)
The involvement of monoamines is generally accepted
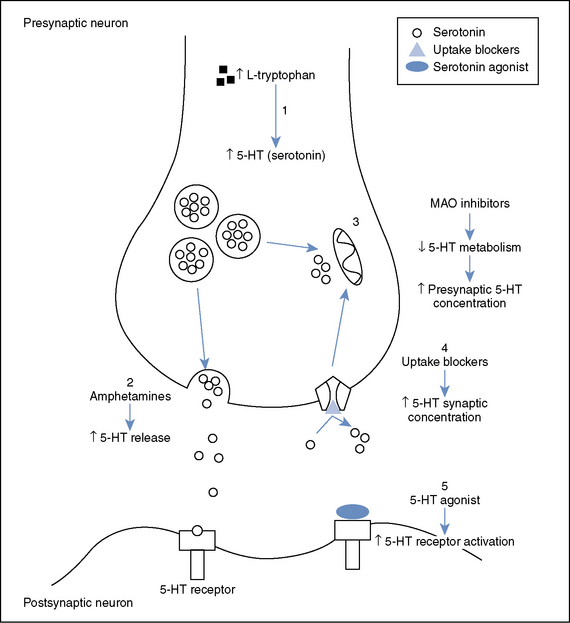
The Monoamine Theory of depression suggests that depression results from a functional deficit of monoaminergic (NorA and/or 5-HT) transmission in the CNS. It is based on the ability of most antidepressant drugs to facilitate monoaminergic transmission. The theory is supported by the fact that pharmacological manipulation of monoamine transmission remains the most successful therapeutic approach to treating depression. However, the theory is, at best, an incomplete explanation of the pathogenesis of depression. It cannot explain why not all drugs that increase amine concentrations (such as amphetamines or cocaine) are antidepressants or the reason for therapeutic lag between initiation of antidepressant treatment and perceived improvement of symptoms.
📚 Read/Explore
There are many antidepressants available
There are many antidepressant drug classes with different modes of action. At this stage, we will focus on monoamine uptake inhibitors and monoamine oxidase inhibitors:
- Monoamine uptake inhibitors
- TCAs
- SSRIs
- SNRIs
- Monoamine Oxidase inhibitors
- MAOIs and RIMAs
- Other antidepressants
- Agomelatine
- Bupropion
- Mianserin
- Mirtazapine
- Reboxetine
- Vortioxetine.
📚 Read/Explore
Monoamine Uptake Inhibitors increase the amount of monoamine available in synaptic cleft for neuronal transmission
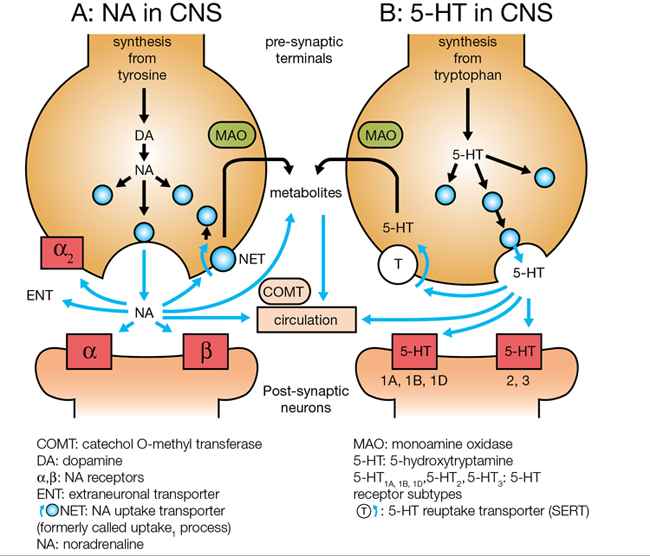
Monoamine uptake inhibitors increase the availability of NorA and/or sertraline in the synaptic cleft by binding to (and inactivating) their respective reuptake transporters. Noradrenaline Transporter (NET) is responsible for the reuptake of NorA whilst 5-HT Transporter (SERT) is responsible for the reuptake of 5-HT. When monoamine uptake inhibitors block the actions of NET and/or SERT, the amount of extracellular neurotransmitter within the synaptic cleft increases and amine transmission improves.
Selective Serotonin Reuptake Inhibitors (SSRIs)
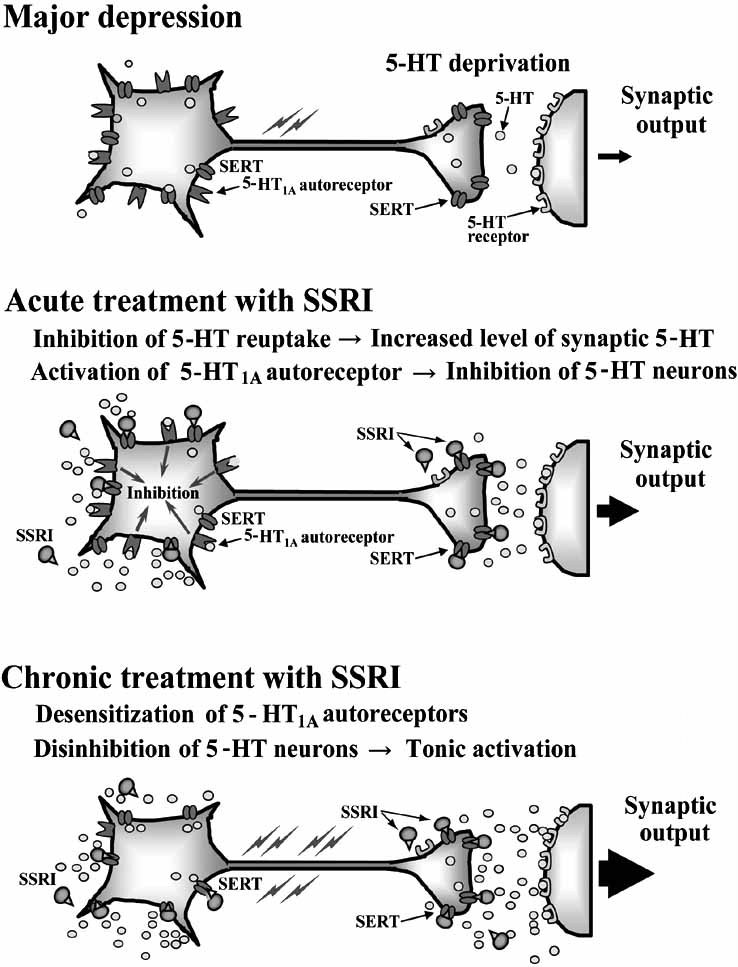
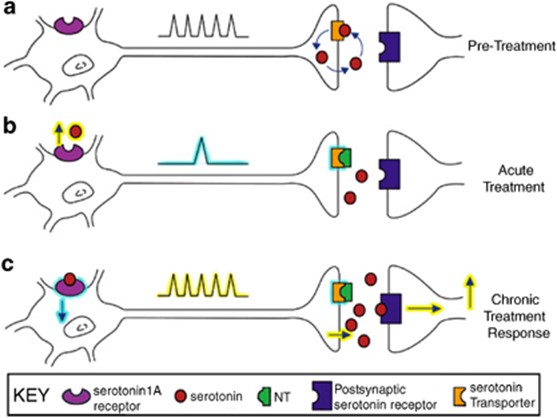
Selective Serotonin Reuptake Inhibitors (SSRIs) are the most commonly prescribed group of antidepressants despite being less effective than TCAs for severe depression. This is due to their favourable side effect profile (no anti-muscarinic adverse effects) and improved safety profile (safer in overdose). They are indicated for depression, generalised anxiety disorder, eating disorders and obsessive-compulsive disorders. They are all highly selective for 5-HT by blocking SERT. An increase in the amount of 5-HT allows for more 5-HT in the synaptic cleft to bind to post synaptic receptors. Chronic enhancement of 5-HT bioavailability produces numerous neuroadaptive changes also. There are many 5-HT receptor subtypes. Stimulation of the 5-HT1A autoreceptor is thought to be the most important for therapeutic effect (increase in mood). Most adverse effects of SSRIs are due to stimulation of postsynaptic 5-HT2 and 5-HT3 receptors. These medications specifically target serotonin (5-HT) uptake over noradrenaline, making them less likely than tricyclic antidepressants (TCAs) to cause anticholinergic side effects and are considered safer in cases of overdose. Unlike monoamine oxidase inhibitors (MAOIs), they do not trigger “cheese reactions” and are also used to manage anxiety disorders and premature ejaculation. Vortioxetine, a novel SSRI, acts on several serotonin receptors: it is an agonist at 5-HT1A, a partial agonist at 5-HT1B, and an antagonist at other receptors, such as 5-HT3A.
Individual responses to different SSRIs may vary, which could be due to other pharmacological actions. For example, fluoxetine also functions as a 5-HT2C antagonist, similar to other non-SSRI antidepressants like mirtazapine, while sertraline slightly inhibits dopamine reuptake. Escitalopram, the S-isomer of citalopram, lacks the antihistamine and CYP2D6 inhibitory actions present in the R-isomer.
Drug examples of SSRIs include:
- Fluoxetine
- Fluvoxamine
- Paroxetine
- Sertraline
- Citalopram
- Escitalopram
- Vortioxetine.
SSRIs are well absorbed when administered orally, with most having a half-life of 18-24 hours. Fluoxetine has a significantly longer half-life (24-96 hours). Paroxetine and fluoxetine should not be combined with TCAs as they inhibit their metabolism via CYP2D6, potentially leading to toxicity.
Adverse Effects
Common side effects include nausea, anorexia, insomnia, decreased libido, and difficulty achieving orgasm. These effects stem from increased extracellular serotonin levels, which overstimulate postsynaptic 5-HT receptors. Overstimulation of certain serotonin receptors, such as 5-HT2, 5-HT3, and 5-HT4, can lead to adverse effects. The therapeutic and adverse outcomes depend on the specific 5-HT receptor and its location in the brain.
Abrupt discontinuation or rapid tapering of SSRIs can lead to withdrawal symptoms like increased anxiety and agitation, which likely result from adaptive changes in the brain. To mitigate these effects, a gradual reduction in dosage is recommended.
Drug Interactions
When combined with MAOIs, SSRIs can cause serotonin syndrome, a potentially life-threatening condition characterized by tremor, agitation, hyperreflexia, hyperthermia, and cardiovascular collapse. There have been reports of increased aggression and occasional violence associated with fluoxetine use, although controlled studies have not confirmed these findings. SSRIs are not typically recommended for children under 18 due to questionable efficacy and the risk of side effects like agitation, insomnia, and aggression. There is also concern about the potential for increased suicidal thoughts in this age group, though SSRIs, particularly fluoxetine, are increasingly being prescribed to younger patients.
Although SSRIs generally have fewer side effects than TCAs, meta-analyses indicate that there is little difference in overall efficacy between the two classes (Cipriani et al., 2018). SSRIs are also safer in overdose compared to TCAs, though they can prolong the QT interval, increasing the risk of ventricular arrhythmias and sudden death.
SSRIs are used to treat various psychiatric conditions, including anxiety, obsessive-compulsive disorder, and eating disorders.
Serotonin and Noradrenaline Reuptake Inhibitors (SNRIs)
The mode of action of Selective Serotonin and Noradrenaline Reuptake Inhibitors (SNRIs) is similar to the TCAs as they block NET and SERT. They are more specific than TCAs and do not affect various other neurotransmitter receptors.
Drug examples of SNRIs include:
- Venlafaxine
- Desvenlafaxine
- Duloxetine.
As the dose of venlafaxine increases, its therapeutic effect also improves, suggesting that its mild inhibition of noradrenaline reuptake may enhance the inhibition of serotonin reuptake seen at lower doses, resulting in additional clinical benefits. These medications are effective when taken orally, and extended-release formulations are available to lessen the likelihood of nausea. Venlafaxine, desvenlafaxine, and duloxetine are useful in treating certain anxiety disorders. Desvenlafaxine may help manage perimenopausal symptoms like hot flashes and insomnia. Duloxetine is also prescribed for conditions such as neuropathic pain, fibromyalgia, and urinary incontinence.
Both venlafaxine and duloxetine are metabolized by the enzyme CYP2D6, with venlafaxine being converted into desvenlafaxine, which has a stronger effect on inhibiting noradrenaline reuptake. Common side effects, primarily due to increased activation of adrenoceptors, include headache, insomnia, sexual dysfunction, dry mouth, dizziness, sweating, and reduced appetite. Overdose symptoms often involve central nervous system depression, serotonin syndrome, seizures, and cardiac conduction issues. Additionally, duloxetine has been associated with liver toxicity and is not recommended for individuals with liver impairment.
Noradrenaline Reuptake Inhibitors (NRIs)
NRIs inhibit noradrenaline uptake but their efficacy in depression is less than SSRIs and TCAs. Examples include reboxetine and atomoxetine. These are indicated for major depression. However, atomoxetine is approved for the treatment of attention deficit/hyperactivity disorder. The common adverse effects include urinary retention, dysuria, urinary frequency (more common in males), dry mouth, sweating, nausea, constipation, decreased appetite, orthostatic hypotension, increase in diastolic BP, tachycardia, impotence, insomnia, headache, paraesthesia and dizziness. The precaution of using of these drugs should be taken in bipolar disorder, epilepsy and cardiovascular disease.
Tricyclic Antidepressants (TCAs)
The Tricyclic Antidepressants (TCAs) are the ‘prototype’ antidepressant as they were the first major group of drugs successful in treating depression. They are indicated for the treatment major depression and also used in neuropathic pain. TCAs block the reuptake of released NA and 5-HT by blocking both NET and SERT. TCAs vary in their activity and selectivity with respect to inhibition of NorA and serotonin reuptake.
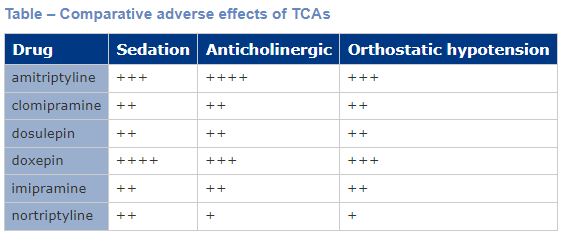
Most TCAs have antagonistic activity at other receptor sites, including adrenergic, muscarinic and histaminergic receptors, which gives rise to many adverse effects. Anti-muscarinic effects include dry mouth, blurred vision, constipation and urinary retention. These effects are strong with amitriptyline and much weaker with desipramine. Postural hypotension, mainly due to α1 adrenoceptor antagonism, occurs with TCAs. The other common side effect is sedation due to histamine1 receptor antagonism, and the long duration of action means that daytime performance is often affected by drowsiness and difficulty in concentrating. The table below shows comparative adverse effects of TCAs. They can also prolong the QT interval and there are many drug-drug interactions with TCAs. Furthermore, they are the most dangerous antidepressant drug class in overdose.
Drug names of TCAs often end in (-mipramine) or (-tryptyline). Examples include:
- Imipramine
- Desipramine
- Amitriptyline
- Nortriptyline
- Clomipramine.
Monoamine Oxidase Inhibitors (MAOIs and RIMAs)
Monoamine Oxidase Inhibitors (MAOIs) are indicated for major depression and some anxiety disorders. There are two MAO enzymes, identified as MAO-A and MAO-B, which are responsible for the degradation of tyramine, NorA, adrenaline, dopamine and 5-HT. MAO-A preferentially inactivates and degrades 5-HT, NorA, adrenaline and dopamine. The inhibition of MAO-A correlates with antidepressant activity as it allows 5-HT, NorA and dopamine stores to build up in the cytoplasm and be readily available for release into the synaptic cleft. MAO-B is more selective for dopamine.
Non-selective and irreversible MAOIs:
- Inhibit both MAO-A and MAO-B for 2-3 weeks.
- Downregulate alpha2 or beta adrenoreceptors and 5-HT receptors.
- Cause significant drug interactions as they impair the metabolism of many amines and drugs.
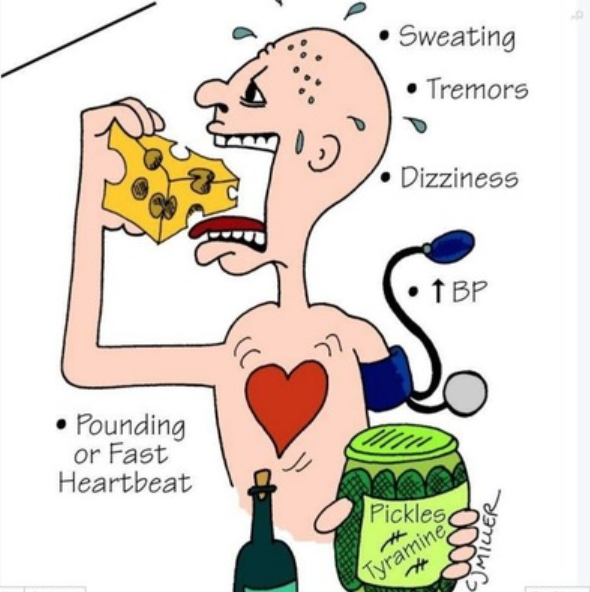
- Concurrent administration with sympathomimeticamines (such as pseudoephedrine) or a diet high in tyramine rich foods can lead to sudden hypertensive crisis and death.
- Overdose carries high risk of fatality.
- Adverse effects include antimuscarinic effects (dry mouth, urinary retention, blurred vision, constipation), weight gain, sleep disturbances and orthostatic hypotension.
- Drug interactions with pethidine, TCAs, SSRIs.
- Drug examples of non-selective, irreversible MAOIs include phenelzine and tranylcypromine.
Competitive and reversible inhibitors of MAO-A (RIMAs)
- Inhibits only MAO-A for 1-2 days.
- MAO-A inhibition correlates with antidepressant activity.
- Less likely to cause drug and food (tyramine) related interactions as short acting.
- Does not require a strict tyramine-free diet but ingestion of large amounts of tyramine containing foods should still in avoided.
- Adverse effects include nausea, headache and insomnia.
- Drug examples of RIMAs include moclobemide.
Monoamine Receptor Antagonists
The examples of the monoamine receptor antagonists used for major depression are mirtazapine, mianserin and trazodone.
Mirtazapine not only inhibits α2-adrenoreceptors but also interacts with other receptors, such as 5-HT2 and 5-HT3, which may play a role in its antidepressant effects while mitigating certain side effects associated with increased serotonin (5-HT) release. By blocking α2-adrenoreceptors, mirtazapine enhances the release of both noradrenaline and serotonin. However, by concurrently inhibiting 5-HT2A and 5-HT3 receptors, it reduces adverse effects linked to these receptors, such as sexual dysfunction and nausea, while still allowing stimulation of postsynaptic 5-HT1A receptors. Additionally, it blocks histamine H1 receptors, leading to sedation, especially at lower doses.
Trazodone functions as a mixed agonist and antagonist of various 5-HT receptors, as well as an antagonist of adrenoceptors and a weak antagonist of histamine H1 receptors. It also acts as a mild serotonin reuptake inhibitor. The combination of 5-HT2A and 5-HT2C receptor antagonism with weak inhibition of serotonin reuptake may result in antidepressant effects with fewer 5-HT2-related side effects, such as sleep disturbances, sexual dysfunction, and anxiety.
Mianserin, another α2-adrenoreceptor antagonist, also blocks H1, 5-HT2A, and α1 adrenoceptors, though it has a reduced impact on serotonin compared to mirtazapine. Mianserin’s potential to cause bone marrow suppression, requiring regular blood tests, has led to a decline in its use in recent years.
Melatonin Receptor Agonists
Agomelatine acts as a strong agonist at MT1 and MT2 receptors, while exhibiting much weaker antagonism at 5-HT2A, 2B, and 2C receptors (approximately 1000 times less potent). It has a short biological half-life and is less likely to cause the side effects commonly seen with other antidepressants.
Typically prescribed for the treatment of severe depression, it is taken once daily, usually at bedtime, and may help regulate circadian rhythm disturbances often linked to depression. However, there have been some reports of hepatotoxicity, so it is contraindicated in individuals with liver conditions
📺 Watch: Treating Depression with Antidepressants
📚 Read/Explore
The risk of serotonin syndrome increases with the dose and number of serotonergic drugs
Excessive stimulation of 5-HT2A by serotonergic drugs can cause serotonin syndrome (serotonin toxicity). The risk of serotonin syndrome increases with the dose and number of serotonergic drugs administered. Examples of drugs which increase serotonin levels include SSRIs, SNRIs, TCAs, MAOIs, fentanyl, tramadol, sumatriptan, dextromethorphan and some complementary medicines (eg St John’s wort). See the table below from the AMH which outlines which drugs that may contribute to serotonin toxicity.
Serotonin syndrome is characterised by confusion, delirium, diarrhoea, tremor, incoordination, sweating, fever and shivering. If left untreated, severe serotonin syndrome can lead to unconsciousness and death. The treatment of serotonin syndrome includes withdrawal of the causative agent(s) and administration of cyproheptadine, a sedating antihistamine with antiserotonin activity.
📺 Watch the following lecture on the management of depression (20 minutes)
Lecture Material:
MP_W9_Lecture_CNS_Antidepressant
Summary
- Two main antidepressant drug classes we have discussed include monoamine uptake inhibitors (TCAs, SSRI, SNRIs) and MAOIs (and RIMAs) but there are many other antidepressant drugs.
- Monoamine uptake inhibitors block NET and/or SERT which increase the amount of NorA and/or 5-HT in the synaptic cleft.
- MAOIs are either selective for MAO-A or non-selective for both MAO-A and MAO-B. The inhibition of MAO-A is responsible for the build up of NorA and 5-HT in the cytoplasm which correlates to antidepressant effects. Non-selective MAOIs are long acting and have a greater propensity for drug and food interactions.
- Overall, these antidepressant drug classes enhance monoaminergic transmission and allow for long term, chronic adaptive changes to occur.
- Although there is similar efficacy between the antidepressant drug classes, SSRIs/SNRIs are safer and better tolerated than TCAs and MAOIs.
- Serotonin syndrome can occur with overdose or co-administration of multiple serotonergic agents. Symptoms are characterized by mental state changes, GI effects, neuromuscular hyperactivity and autonomic instability.
💡Learning Activity
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.