10.4 Opioid Agonists (Narcotics)
Opioid Agonists (Opioids)
Opioids, or narcotics from the greek narke for stupor/numb, have been used for thousands of years for their analgesic and euphoric properties. Morphine is a naturally occurring alkaloid found within the sap of unripe seedpods of the opium poppy. A variety of natural, semi-synthetic and synthetic opioids are in clinical use.
Tasmania grows around 50% of the global pharmaceutical opium poppy crop used for the production of natural and semi-synthetic opioids.

Opioids are very commonly prescribed medications for the management of pain and some other common conditions.
Examples of opioids routinely used in clinical practice include:
- Morphine
- Codeine
- Oxycodone
- Fentanyl
- Tramadol
- Tapentadol
- Buprenorphine (partial agonist)
- Methadone
- Loperamide (not for analgesia)
There are also endogenous opioids within the body:
- Endorphins
- Enkephalins
- Dynorphin
Opioid Mechanism of Action
Watch the following video on opioids:
Opioids exert their effects by binding to opioid receptors at various sites throughout the body. There are four main types of opioid receptors:
- Mu (MOPr)
- Delta (DOPr)
- Kappa (KOPr)
- Opioid Like Receptor (NOPr)
The analgesic effects of opioids are primarily due to mu receptors (MOPr).
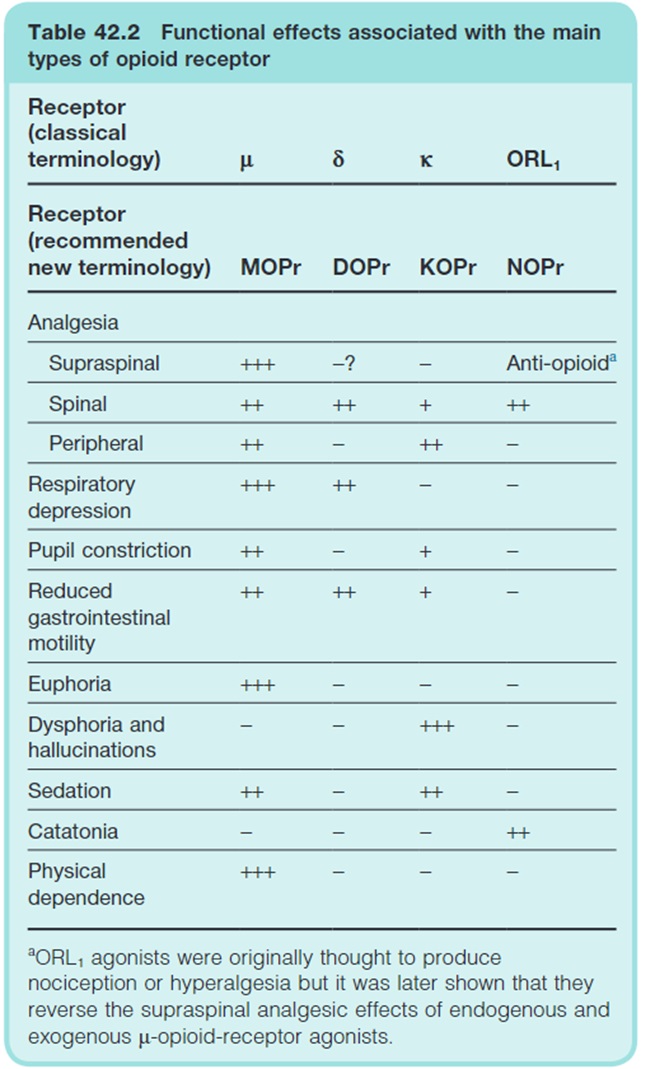
You will note in the table above that opioids are associated with a variety of non-analgesic effects. Of particular importance are respiratory depression, reduced GI motility, euphoria, dysphoria/hallucinations, sedation and physical dependence – these will be discussed later.
Thinking back to the pain pathways there are three potential sites of action for opioids. At the peripheral nociceptor, in the dorsal horn and in the descending inhibitory pathways.
The effects of opioids are best described in the dorsal horn, periaqueductal grey and rostral ventromedial medulla.
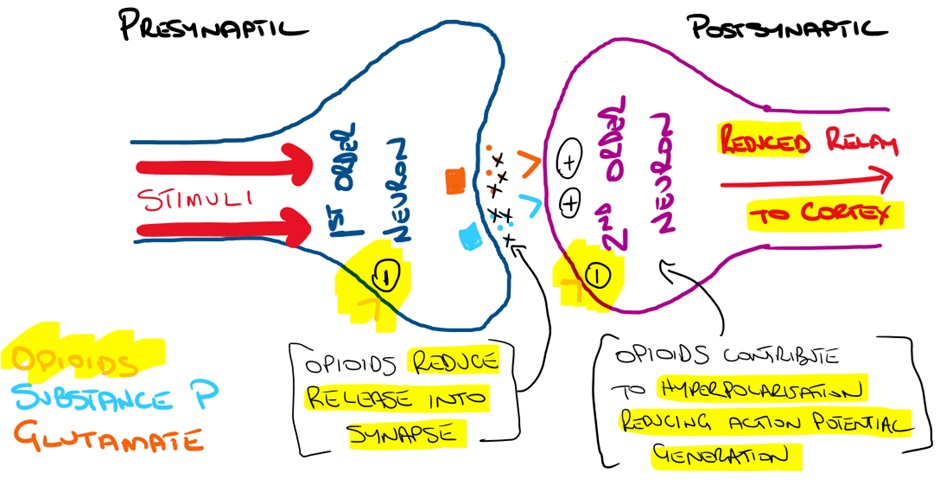
Dorsal horn effects
Exogenous (and endogenous) opioids binding to opioid receptors inhibit the release of excitatory neurotransmitters within the dorsal horn. This reduces afferent relay of pain signals from the 1st order to 2nd order neurons (and ultimately the somatosensory cortex).
When opioids stimulate the presynaptic opioid receptor they inhibit Ca2+ entry (N-type channel) and increase the outward movement of K+. There is a decrease of cAMP through G protein coupled inhibition of adenylate cyclase (AC). As a result, there is a decrease in the release of excitatory neurotransmitters (such as glutamate and substance P).
When opioids stimulate the postsynaptic opioid receptor the outward movement of K+ leads to hyperpolarisation. This reduces the likelihood of an action potential and reduces neuronal firing leading to reduced transmission to the thalamus and somatosensory cortex.
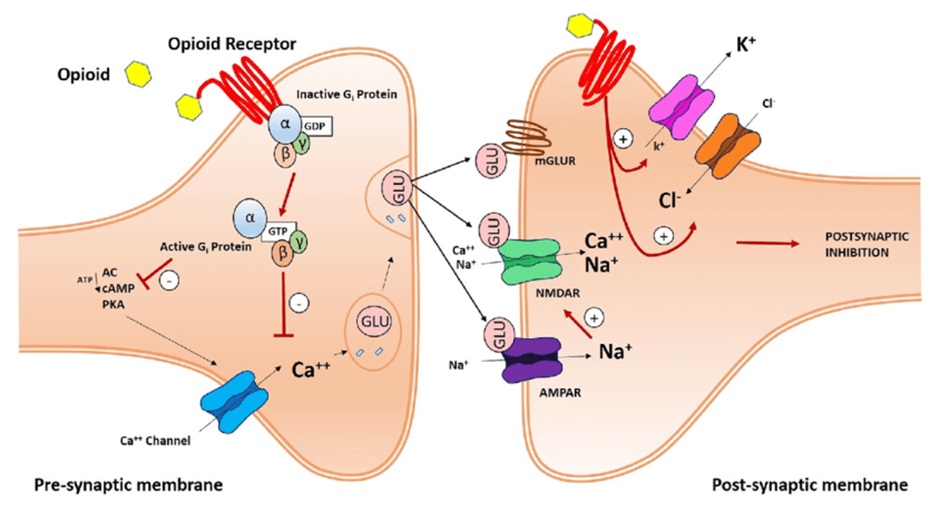
In summary, opioids binding to opioid receptors results in the following:
- inhibition of adenylyl cyclase
- closure of voltage-gated calcium channels
- opening of potassium channels and membrane hyperpolarisation
Brainstem effects
Opioids indirectly stimulate activation of inhibitory pain pathways within the periaqueductal grey (PAG), rostral ventromedial medulla (RVM) and perhaps the locus coeruleus along with other higher sites via inhibition of GABAergic neurons.
You will recall that GABAergic neurons release the neurotransmitter GABA. GABA is an inhibitory neurotransmitter. Within the PAG and RVM, GABA therefore reduces activation of the inhibitory descending pathways.
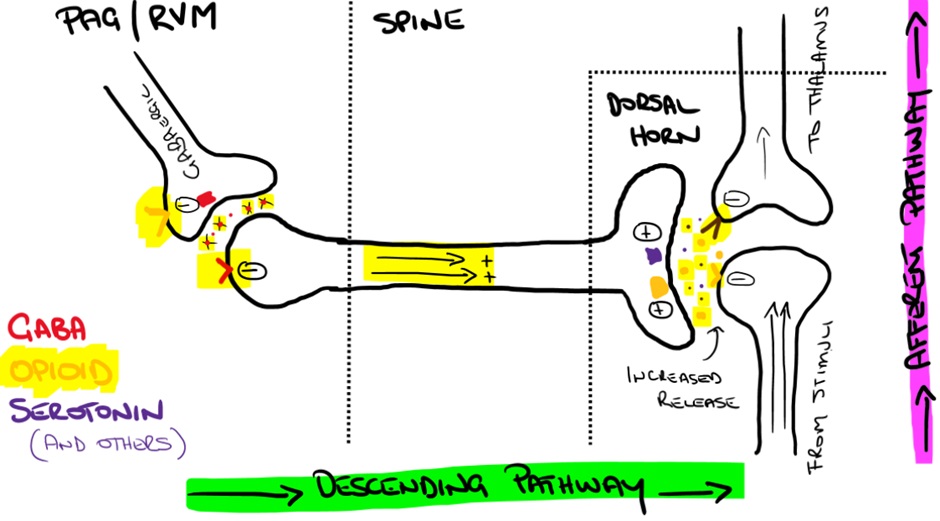
In response to opioid biding, presynaptic opioid receptors on GABAergic neurons inhibit the release of GABA leading to reduced inhibition (or a net increase) of descending pain inhibiting pathways (causing analgesia).
Increased output in the inhibitory descending pathways from the PAG and RVM lead to the release of various neurotransmitters (including enkephalins) at the dorsal horn where they inhibit the relay of signals between 1st order and 2nd order afferent neurons.
Confused? Remember double negatives.
Tolerance and Dependence
Tolerance develops when an opioid is given repeatedly over a period and the effectiveness of the drug (at a given dose) decreases. Patients will often require higher doses of the drug to achieve the same effect as tolerance develops. Tolerance begins to develop with the first dose but typically takes 7-10 days to become apparent.
Tolerance is more likely to develop to the analgesic and euphoric effects, nausea and vomiting and sedation. Bradycardia, miosis and constipation often persist during therapy.
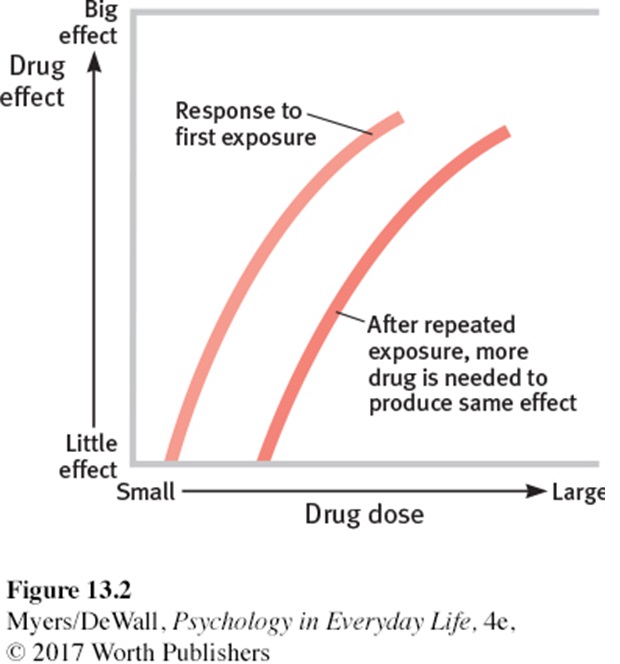
Dependence to opioids causes an abstinence syndrome when the opioid is withdrawn. Symptoms include flu-like symptoms, fever, sweating, lacrimation, yawning, piloerection, diarrhoea, anxiety, and hostility.
Although the risk of dependence may be less with some opioids; all opioids can cause dependence, and this must be considered when initiating opioid therapy. Up to 35% of chronic non-cancer pain opioid users may meet the criteria for opioid-use disorder.
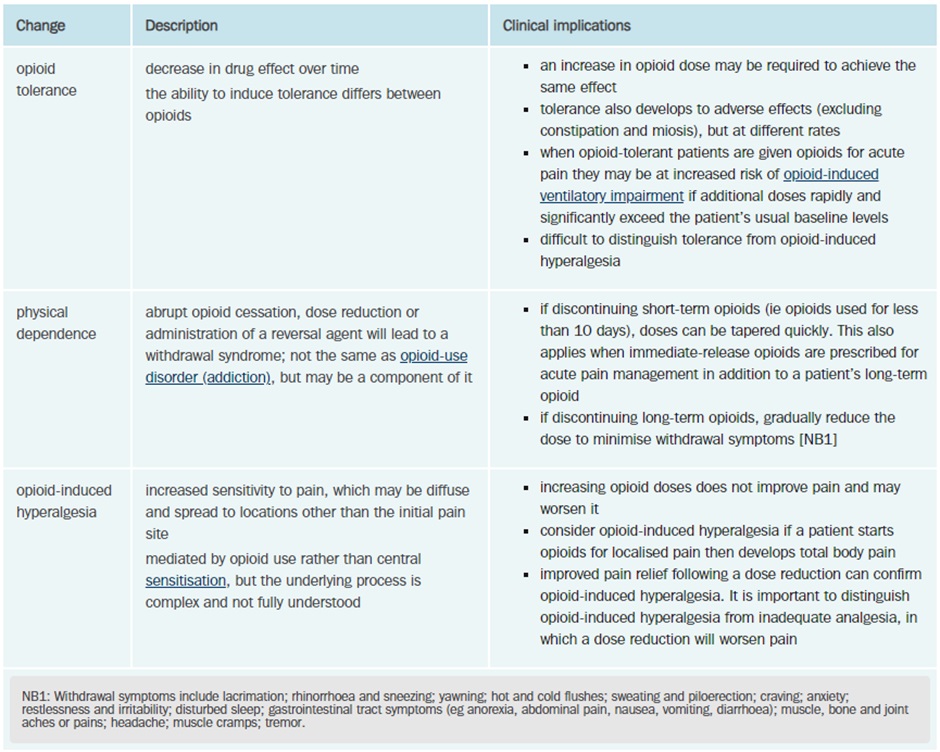
Opioid-induced Ventilatory Impairment (Respiratory Depression)
The risk of respiratory impairment is highest in opioid-naïve patients, those with organ dysfunction that may impair metabolism (renal or hepatic), the elderly or very young and those with respiratory conditions such as obstructive sleep apnoea, asthma or obstructive airways diseases as well as the use of other sedating substances (eg alcohol, gabapentinoids, cannabis).
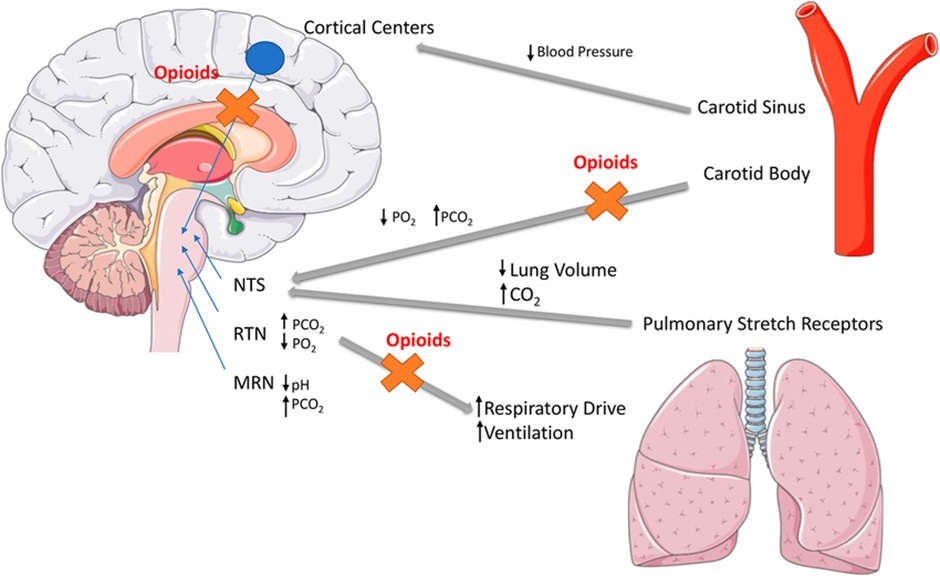
Opioids binding to the MOPr lead to a direct depressant effect on resp rhythm generation, depression of the ventilatory response to increased CO2 and reduce carotid/aortic body chemosensors response to hypoxia. Exogenous opioids that are full agonists at MOPr have the highest risk of respiratory impairment however it can occur with any opioid.
Naloxone is a potent opioid receptor antagonist and is used to reverse respiratory depression in overdose. Naloxone has a shorter half-life than many opioids and repeated doses may be required to prevent the return of opioid-induced ventilatory impairment.
Opioids also inhibit the cough reflex and are used clinically for cough suppression (morphine, dextromethorphan and dihydrocodeine).
Constipation
Tolerance to many of the side effects of opioids develops relatively quickly however little if any tolerance develops to the constipating effects of opioids. Constipation occurs due to activation of presynaptic inhibitory MOPr on enteric neurons which decreases gastric motility. All patients prescribed more than a couple of doses of opioids should be prescribed laxatives.
Administration of oral naloxone (has very low bioavailability due to high first pass metabolism) with oral opioids (eg oxycodone – Targin) produces modest reductions in constipation.
Administration of intravenous methylnaltrexone (an opioid antagonist that doesn’t penetrate the blood-brain-barrier) can be used to reverse severe opioid induced constipation.
Loperamide, an opioid, is used for the treatment of diarrhoea and to reduce stoma output (ie we are using the constipation side effect therapeutically). Although loperamide is an opioid, it doesn’t readily cross the blood-brain-barrier and therefore does not display analgesic or euphoric effects at therapeutic doses.
Tramadol and Tapentadol (the opioids that also act like adjuvants)
Tramadol and tapentadol are not particularly potent agonists at the MOPr but they also inhibit reuptake of serotonin (tramadol) and noradrenaline (tramadol and tapentadol). These additional non-MOPr mediated effects may contribute to benefits in neuropathic pain. Tramadol has more data than tapentadol in neuropathic pain.
🎥 Watch this video
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.
