12.1 Neurological Drugs – Antiepileptics
Neurological Drugs – Antiepileptics
In this topic, we will look at the pharmacology of antiepileptic drugs. We will explore the nature and types of epilepsy and the mechanisms of actions and clinical uses of antiepileptic drugs. We will examine the interesting pharmacokinetics of phenytoin and explain the rationale of therapeutic drug monitoring with the use of phenytoin.
In epilepsy there is an imbalance between activity in stimulatory and inhibitory neural pathways
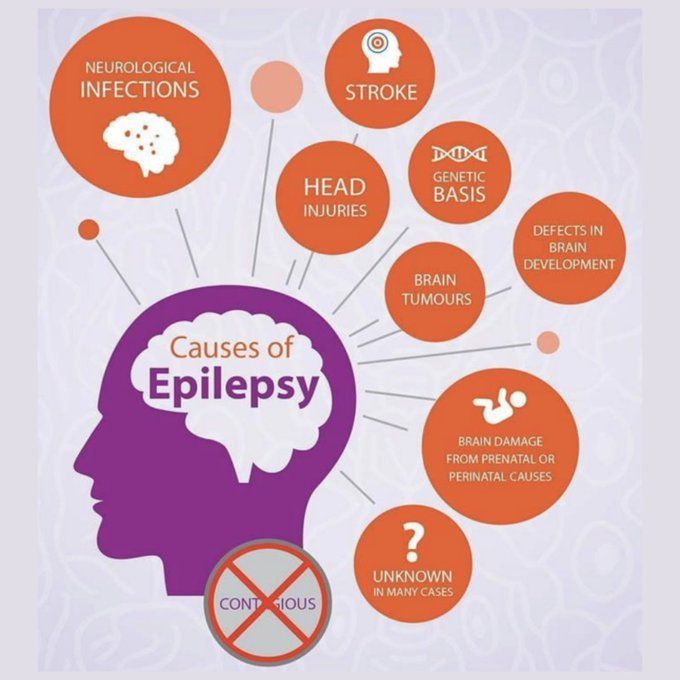
Epilepsy is one of the most common chronic neurological diseases and affects approximately 50 million people worldwide (0.5-1% of the population). It is a term used to define a group of neurological disorders, characterised by recurrent seizures. A seizure is a transient alteration in behaviour, sensation, awareness or movement. It is thought that disorderly discharge in neuronal pathways in the brain is responsible for seizure activity. In epilepsy there is an imbalance between activity in stimulatory vs inhibitory neural pathways and there are changes to membrane potentials of neural cells. The following points underpin the pathophysiology of seizures and epilepsy:
- Enhanced excitatory amino acid transmission (glutamate)
- Impaired inhibitory transmission (GABA)
- Abnormal electrical properties of the affected cells
Due to an alteration in membrane potential certain neurons can become abnormally hyperactive and hypersensitive to changes in their environment. It becomes easier for the neurons to act spontaneously (this means seizure threshold is lowered) as the resting membrane potential is changed. These neurons can act autonomously and result in the release and propagation of an action potential and they can recruit nerve cells in adjacent or distant areas of the brain.
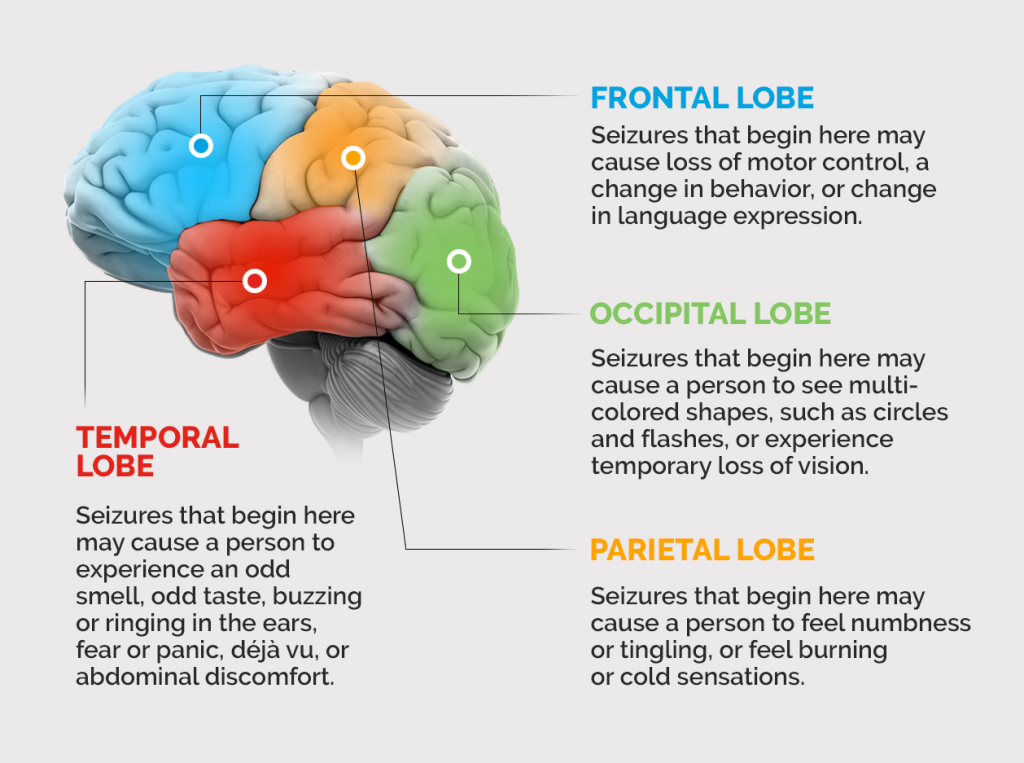
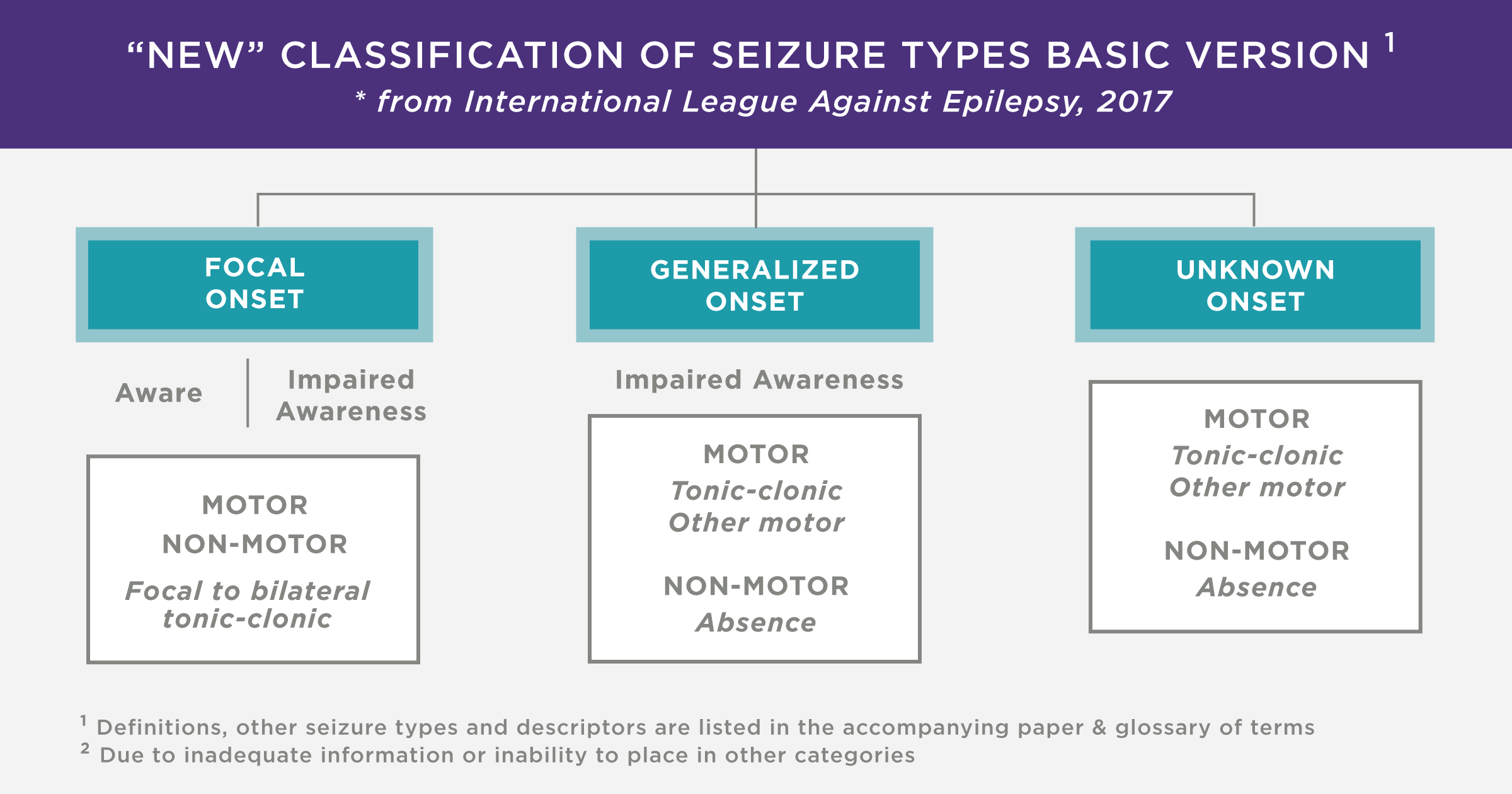
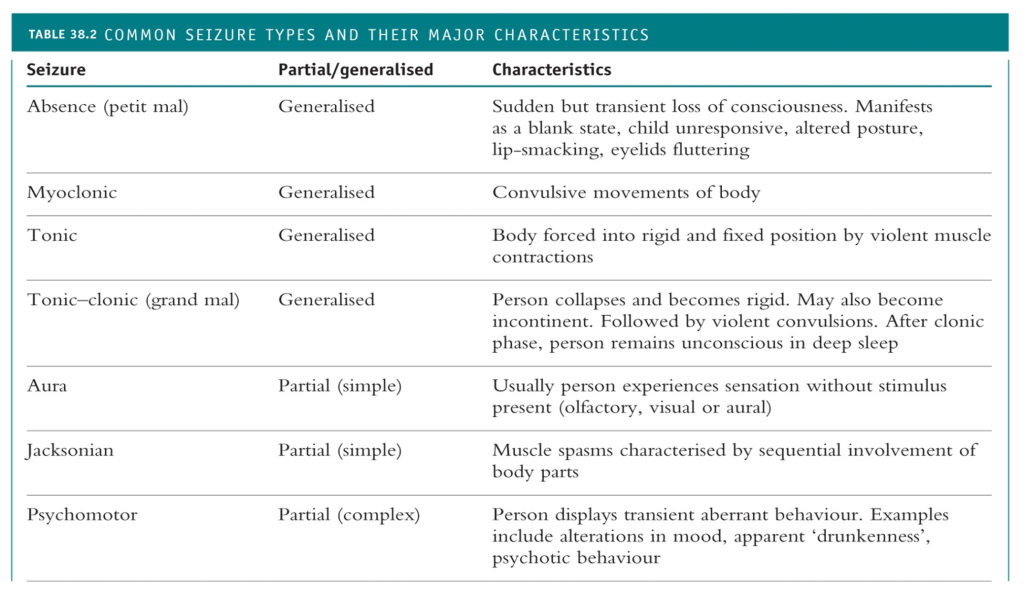
When the origin of abnormal electrical activity in the brain is localised to part of the brain it is called a focal onset (partial) seizure. There are focal aware (simple partial) and focal impaired awareness (complex partial) seizures. In comparison, inappropriate neuronal firing that originates simultaneously from bilateral hemispheres of the brain is referred to as a generalised onset seizure. The two main types of generalised onset seizures are motor (tonic-clonic) and non-motor (absence) seizures. It is possible for a partial seizure to become secondarily generalised.
The epileptogenic focus (the area of the brain from which the disorderly neuronal discharge begins) and the extent of spread determines the symptoms of the seizure. If neurons in the motor cortex are involved then involuntary movements may be present whereas if neurons in the reticular system (brain stem) are involved then a patient might experience altered consciousness. It is important to note that not all seizures involve convulsions and some seizures manifest as brief lapses of attention or sensory and behavioural phenomena.
How do AEDs work?
📺 Watch this video on Pharmacology of AEDs:
The aim of treatment in epilepsy is to inhibit the abnormal, inappropriate neuronal firing in the brain. It does not address or treat the underlying cause of epilepsy in the patient. The mechanisms of action of currently marketed antiepileptic drugs (AEDs) are not fully understood however main mechanisms of action of AEDs are thought to:
- Altering the movement of sodium ions through membrane channels/blocking sodium channels
- Inhibition of calcium channels
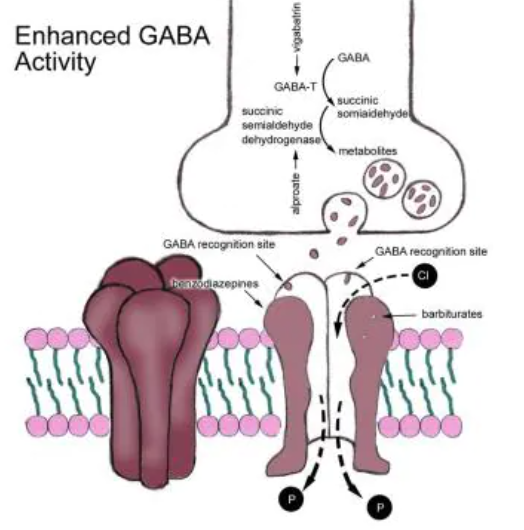
- Enhance the action of inhibitory transmitters such as GABA
- Inhibit the action of excitatory transmitters such as glutamate.
1. Altering the movement of sodium ions through membrane channels/blocking sodium channels
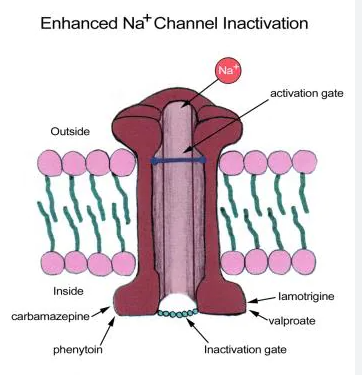
Many antiepileptic drugs reduce membrane excitability by acting on voltage-dependent sodium channels. These AEDs block “use dependent” sodium channels, which means they block high frequency discharge (seizure activity) without interfering with the low frequency firing of neurons. AEDs that alter the movement of sodium ions through membrane channels reduce the rate of recovery which means the cells are inactive for longer and this prevents repeated neuronal firing. They can also increase sodium excretion during the refractory period which also drives cells way from reaching threshold.
2. Inhibition of calcium channels
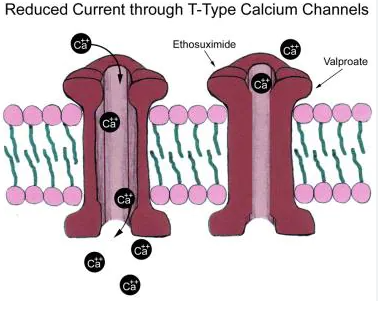
Drugs that are used to treat absence seizures have the ability to block T-type low-voltage-activated calcium channels. Valproate, ethosuximide and zonisamide are examples of AEDs that stabilise neuronal membranes by blocking T-type calcium channels.
3. Enhance the action of inhibitory transmitters such as GABA
Several AEDs increase extracellular levels of GABA. In doing so, the activation of GABAA receptors, the opening of chloride channels and the inhibitory effects of GABA in the CNS are potentiated. Valproate works partly by stimulating glutamic acid decarboxylase (the enzyme responsible for GABA synthesis) and inhibiting GABA transaminase (the enzyme responsible for GABA breakdown). Tiagabine inhibits the GABA transporter GAT1 which prevents the removal of GABA from the synapse.
4. Inhibit the action of excitatory transmitters such as glutamate
Excessive excitatory neurotransmission mediated by glutamate and other excitatory amino acids can contribute to seizure activity. Lamotrigine is an example of an AED that inhibits glutamate release while topiramate blocks AMPA receptors. Perampanel is an example of an AED that is a non-competitive antagonist of glutamate receptors.
📚 Read/Explore
Read: “Antiepileptic Drugs” in Chapter 46 Nervous System (page 580-590) in Rang and Dale’s Pharmacology 9th Ed.
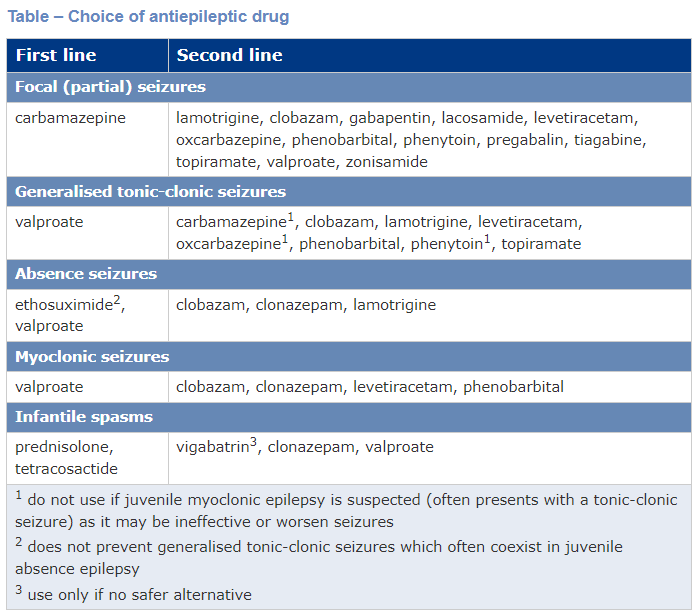
AEDs can be classified by first generation (older) or second generation (newer) drugs. Otherwise, they can be classified as those used first line or second line in treatment regimens.
Epilepsy and Drug Targets for Epilepsy Lecture
 Watch the following lecture on Epilepsy and Drug Targets for Epilepsy (10 minutes)
Watch the following lecture on Epilepsy and Drug Targets for Epilepsy (10 minutes)
Pharmacology of Antiepileptic Drugs (AEDs)
Valproate
- MOA is not fully established. Inhibits voltage-gated sodium channels, causes small reduction in T-type calcium channels and enhances inhibitory effects of GABA (increases synthesis of GABA and inhibits its breakdown).
- Broad spectrum so can be used for all seizure types.
- Narrow therapeutic range but TDM is not helpful.
- Adverse effects include nausea, vomiting, increased appetite, weight gain, thinning or loss of scalp hair, drowsiness, dizziness, menstrual irregularities, rash and hepatotoxicity.
- Teratogenic.
Carbamazepine
- Blocks sodium channels which prevents neuronal discharges, reduces synaptic transmission and reduces spread of seizure discharge.
- Useful to manage focal impaired awareness (complex partial) seizures.
- Causes many drug-drug interactions. An enzyme inducer including auto=induce it’s metabolism
- Adverse effects include severe hypersensitivity and skin reactions, drowsiness, dizziness, blurred vision, abdominal pain, nausea, vomiting, diarrhoea, constipation, blood dyscrasia.
- Teratogenic.
Ethosuximide
- Inhibits T-type calcium channels which decreases calcium conductance in thalamic neurons.
- Used for absence seizures.
- Adverse effects include anorexia, nausea, vomiting, epigastric pain, weight loss, drowsiness, dizziness.
Phenytoin
- Oldest non-sedating antiepileptic agent, now considered as 2nd line agent for epilepsy
- Blocks sodium channels which prevents neuronal discharges, reduces synaptic transmission and reduces spread of seizure discharge.
- Adverse effects include nystagmus and confusion (dose related); hirsutism and gingival hyperplasia (chronic use)
- Pharmacokinetics: Narrow therapeutic index, oral bioavailability varies with formaulations, metabolism is saturable with variable half-life ranging from 12-36 hours. Follows non-linear PK, binds extensively (99.9%) to albumin, also induces CYP enzymes. More information on phenytoin PK is provided at the end of this module.
Gabapentin and Pregabaline
- M/A: Not fully understood; blocks presynaptic voltage-dependent Ca2+ channels reducing calcium influx and release of excitatory neurotransmittersn (glutamate); Raises GABA levels
- Indicated for refractory partial seizures (as adjunct) and neuropathic pain (trigeminal neuralgia)
Lamotrigine
- Broad spectrum, widely used
- Believed to block voltage-gated sodium channels and voltage-gated calcium channels as well as inhibit the release of excitatory transmitters like glutamate and aspartate.
- Main side effects are nausea, dizziness, ataxia and hypersensitivity (mild to severe rashes).
- Indicated alone or as adjunct for partials/tonic-clonic/absences
Topiramate
- Mechanism: Inhibits voltage-gated Na+ channels, enhances GABAA-Cl- conductance, activates hyperpolarising K+ current, reduces action of AMPA/kainate receptors
- Adverse effects include somnolence, headache, dizziness, confusion, amnesia, impaired concentration, depression.
- Indicated alone or as add-on for refractory partial seizures
Levetiracetam
- MOA is not fully established, may modulate neurotransmission by binding to synaptic vesicle protein 2A
- Used as adjunct for refractory partial seizures/uncontrolled generalised tonic-clonic seizures
Lacosamide
- Enhances slow inactivation of voltage-gated sodium channels.
Zonisamide
- Blocks some sodium and T-type calcium channels and modulates GABA-inergic inhibition.
Vigabatrin
- Irreversible inhibitor of GABA transaminase, the enzyme that inactivates GABA. This allows a build up of GABA in the synapse and promotes GABA-inergic transmission.
- Used last line as has a specific adverse effect on vision (can cause irreversible visual field constriction in 20-40% of patients).
Tiagabine
- Inhibits neuronal GABA reuptake, increasing GABA-mediated inhibition.
- Used for focal (partial) seizures with or without secondary generalisation.
- Adverse effects include nausea, vomiting, diarrhoea, dizziness, fatigue, somnolence and visual field defects.
Phenytoin has very interesting pharmacokinetics and requires TDM
Phenytoin prevents repetitive neuronal discharge by blocking voltage-dependent and use-dependent sodium channels. It stabilises the inactive state of the sodium channel and prolongs the neuronal refractory period. Phenytoin is effective in treating all types of epilepsy except generalised non-motor (absence) seizures. It can also be used in status epilepticus and for seizure prophylaxis in neurosurgery. Adverse effects of phenytoin include nausea, vomiting, insomnia, sedation, nystagmus and gingival hypertrophy.
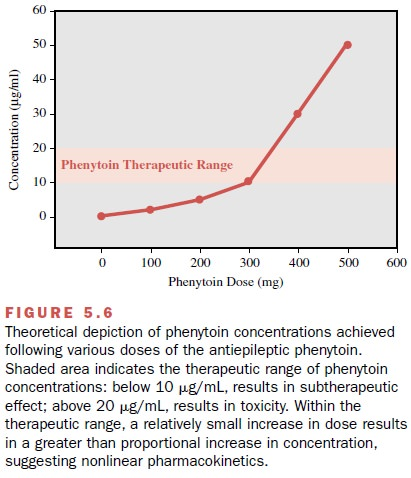
Phenytoin has non-linear pharmacokinetics which means a small dose change may result in a disproportionately large change in plasma concentration of phenytoin. This is because the enzyme system involved in phenytoin metabolism gradually becomes saturated and so as the dose increases there is a decrease in the rate of elimination of phenytoin. Phenytoin also has a narrow therapeutic index (NTI), meaning that there is only small window in which plasma concentrations are safe and effective for the patient. The usual therapeutic range of phenytoin is:
- Total phenytoin 10–20 mg/L (40–80 micromol/L)
- Free phenytoin 1–2 mg/L (4–8 micromol/L)
Even though there is limited correlation between therapeutic efficacy and the plasma concentration of phenytoin, therapeutic drug monitoring (TDM) is important to ensure the safety of the patient. Scenarios where monitoring levels may be clinically useful include:
- Treatment initiation
- Dose or brand changes
- Signs and symptoms of toxicity in a patient
- Assessing patient compliance
- Physiological changes in a patient (eg renal impairment or uremia)
- Initiation or cessation of interacting drugs
📚 Read/Explore
Antiepileptic Drugs Lecture
 Watch the following lecture on pharmacology of Antiepileptic Drugs (19 minutes)
Watch the following lecture on pharmacology of Antiepileptic Drugs (19 minutes)
 Lecture Notes
Lecture Notes
Summary
- Epilepsy is a neurological disorder, characterised by periodic seizures.
- During a seizure, neurons fire abnormally. The nerve cell becomes more easily excited as the resting membrane potential changes and spontaneous neuronal activity is more easily triggered.
- Partial seizures occur when abnormal electrical impulses originate from a focal point in the brain while generalised seizures involve the whole brain.
- Antiepileptics target neuronal pathways that involve: inhibitory transmitters such as GABA or glycine, excitatory transmitters such as glutamate or reduce the electrical excitability of cell membranes through use dependent blockade of voltage-gated sodium channels or voltage-gated calcium channels.
- Examples of some antiepileptic drugs include valproate, carbamazepine, ethosuximide, lamotrigine, gabapentin, pregabalin, lacosamide, topiramate, levetiracetam, zonisamide, vigabatrin, tiagabine and phenytoin.
- Phenytoin blocks voltage-gated and use-dependent sodium channels and is used to treat all types of epilepsy except absence seizures.
- Phenytoin has a narrow therapeutic index, non-linear pharmacokinetics and a high degree of protein binding.
- Therapeutic drug monitoring is useful to investigate suspected toxicity or non-compliance with phenytoin but plasma concentrations do not correlate well with efficacy.
💡Learning Activity
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.