1.3 Drugs that affect the Sympathetic Nervous System
John Smithson
Learning Outcomes
Be able to:
- Understand the relationship between the sympathetic nervous system and sympathomimetic agents
- Describe the process of release and inactivation of catecholamines
- Link the receptor activity of the sympathomimetic agents to their clinical use
- Apply knowledge of the sympathetic nervous system and pharmacology of sympathomimetic drugs to predict effect and side effect of these agents.
The Sympathetic Nervous System (SNS)
The second division of the autonomic nervous system is the sympathetic nervous system (remember, the other one was the parasympathetic nervous system).
Stimulation of the alpha (α) and beta (β) receptors by the sympathetic nervous system is almost entirely medicated by the release of noradrenaline (aka norepinepherine or NA) from the post ganglionic neurons or noradrenaline and adrenaline (aka epinephrine in some other countries) from the adrenal medulla after stimulation by the preganglionic neuron. The third neurotransmitter in the SNS is dopamine but in terms of the SNS, dopamine is the precursor to noradrenaline and adrenaline (and also a major neurotransmitter in the central nervous system so worth understanding its context) and won’t be discussed further in this module. Acetylcholine is still the pre-ganglionic neurotransmitter, but it is mostly adrenaline and noradrenaline that is the primary neurotransmitter released from the post-ganglionic neuron (NE only) in the sympathetic nervous system (SNS) and from adrenal medulla (both E and NE). The notable exception is the release of acetylcholine (that interacts with muscarinic receptors) that stimulate sweating and piloerection.
It is important to remember the only neurotransmitter released by a postganglionic nerve in the SNS is noradrenaline. Both adrenaline and noradrenaline are released from the adrenal medulla following innervation by the SNS. The Adrenal Medulla (the Chromaffin cells specifically) is the primary site for the production of circulating dopamine, noradrenaline and adrenaline. Upon stimulation, the adrenal medulla releases a mixture of the two catecholamine’s, approximately 80-85% adrenaline and the remainder noradrenaline.
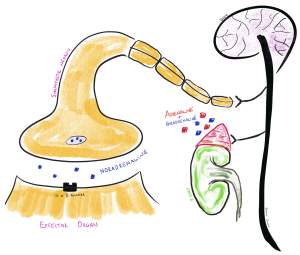
📺 Watch the vodcast on transmission and the role of adrenaline and noradrenaline SNS (1:49 min).
Receptors and Neurotransmitters of the SNS
The primary receptors in the SNS are α1, α2, β1 and β2. (Note: There is also a third type of β receptor – β3 – mainly found in adipose tissue and regulate thermogenesis but less important for this discussion).
All α and β receptors are G-coupled protein receptors which means they cause their cellular response via some sort of second messenger system. The alpha receptors use a range of second messengers and the beta receptors use adenylyl cyclase as the second messenger system. The receptors differ in a number of ways – importantly the relative potency of the different sympathomimetic agents on the receptor and the location of the receptors (target organ). The relative potency and location of the different receptors can explain why we can get different physiological reactions from the different sympathomimetic agents. The exogenous sympathomimetic agents we are talking about are dopamine, noradrenaline, adrenaline, dobutamine and isoprenaline.
| Receptor | Agonist potency order | Important receptor location |
| α1, | Noradrenaline > Adrenaline >> Isoprenaline | Vascular smooth muscle, bronchi smooth muscle, gastrointestinal smooth muscle, uterus, iris |
| α2, | Adrenaline > Noradrenaline >> Isoprenaline | Vascular smooth muscle, gastrointestinal smooth muscle |
| β1 | Isoprenaline > Noradrenaline > Adrenaline | Myocardial tissue |
| β2 | Isoprenaline > Noradrenaline = Adrenaline | Bronchi smooth muscle, vascular smooth muscle, Gastrointestinal smooth muscle, myocardial tissue, skeletal muscle, mast cells |
Note: Bold = important tissue targets
These receptors are located throughout the body in the heart, lungs, smooth muscle of the arterioles, skin, eye, bladder and the gastrointestinal tract. As a generalisation stimulation of:
- α1 receptors cause vasoconstriction, relax the GI tract smooth muscle, increase salivary secretion and stimulate hepatic glycogen breakdown. Probably the most important thing to remember about these receptors is the effect on vascular smooth muscle. Activation of the α1 receptors cause a contraction of the smooth muscle in the small arteries and arterioles and splenic vascular beds (The splanchnic circulation accounts for blood circulation to a large number of vital organs including the small and large intestine, liver, spleen, stomach and pancreas and changes in blood flow to this system can have a significant effect on peripheral vascular resistance and blood pressure … have a read about the splanchnic circulation) and smooth muscle in the large arteries and veins which increases arterial and venous pressure (at the cost of cardiac workload). The rise in venous pressure can cause reflex bradycardia. The mechanism of vasoconstriction is via increased release of intracellular calcium.
- α2 receptors inhibit some neurotransmitter release, promote platelet aggregation, cause smooth muscle contraction and inhibit the release of insulin from the pancreas. Stimulation of the α2 receptors in the gastrointestinal smooth muscle cause a decrease in release of acetylcholine causing relaxation of the gastrointestinal smooth muscle.
- β1 receptors increase heart rate and contractility. This causes a marked increase in cardiac output.
- β2 receptors cause bronchodilation, vasodilatation, relax visceral smooth muscle, promote hepatic glyconeolysis and cause muscle tremor.
📺 Watch the vodcast on the different neurotransmitters and receptors of the SNS (1:49 min).
Synthesis of Noradrenaline and Adrenaline and its Release
Three endogenous neurotransmitters are produced from tyrosine – Dopamine, noradrenaline and adrenaline. These are commonly called catecholamines as they all share a ‘catechol’ group.
In the adrenergic neurons, exogenous tyrosine (tyrosine is only sourced from the diet, there is no production of tyrosine in the body) is converted to DOPA by the cytosolic enzyme tyrosine hydroxylase. DOPA (dihydroxyphenylalanine) is converted to dopamine by DOPA decarboxylase. It is then converted into noradrenaline by the enzyme dopamine β-hydroxylase in the neuronal storage vesicles. There is no conversion of noradrenaline to adrenaline in the adrenergic neurons. The same process occurs in the Chromaffin cells in the adrenal medulla except the noradrenaline is then converted into adrenaline by phenylethanolamine N-methyltransferase.
Knowledge check
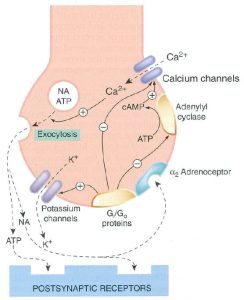
Very little of the produced catecholamine (adrenaline or noradrenaline) is free in cytoplasm in adrenergic nerve terminals or the adrenal medulla. The majority is packaged up into vesicles for release by the adrenergic neuron by the process of exocytosis. Exocytosis occurs in response to an increased concentration of calcium ions that result from the action potential reaching the nerve terminal. This causes the binding of the catecholamine-containing vesicles with the inner surface of the plasma membrane of the nerve.
The release of catecholamine from the adrenergic nerve terminal is mediated by noradrenaline itself. The released noradrenaline interacts with the α2 receptor on the pre-synaptic terminal. You will remember this adrenoreceptor has an inhibitory effect on the release of neurotransmitter (a homotropic interaction). Activation of the pre-synaptic α2 receptor has an inhibitory effect on the calcium channel of the adrenergic neuron thus reducing calcium influx and further exocytosis. See Figure 1.3.2 below – Feedback control of noradrenaline release.
Termination of catecholamine action. Termination of the effect of noradrenaline released by the adrenergic nerve terminal is mainly achieved by reuptake back into the adrenergic neuron (this accounts for approximately 75% of NA released into the synapse – which is taken back into the sympathetic neuron and repackaged into a vesicle ready for release again). The neuronal plasma membrane transporter noradrenaline transporter (NET) is responsible for the majority of the noradrenaline transport back into the adrenergic nerve. Extra-neuronal monoamine transporter (EMT) on the nearby non-neuronal cells is responsible for much of the remaining of the NA not dealt with by NET.
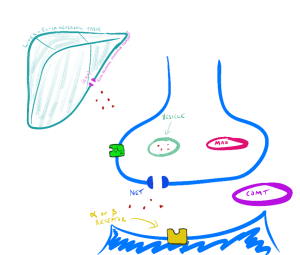
Intracellular metabolic deactivation of catecholamine’s action is undertaken mainly by two enzymes: monoamine oxidase (MAO) and catechol-O-methyl transferase (COMT). The MAO enzyme has two major subtypes MAOA and MAOB. Both are bound to the surface of mitochondria in the adrenergic neuron but are present in other tissue including the liver and intestinal epithelial. As you will discover later in this subject, these metabolic enzymes are important targets for some commonly used antidepressants. The MAO enzymes play a role in maintaining a lower level of catecholamine in the cytosol in the adrenergic nerve. Where these enzymes are inhibited, available intracellular stores of adrenaline and noradrenaline are elevated. MAOA and MAOB are important enzymes for limiting the effect of circulating catecholamine’s but are not the major mechanism. Like the termination of catecholamine action in the adrenergic neuron, the majority of circulating adrenaline is captured by EMT and circulating noradrenaline by NET. COMT plays a lesser role in limiting the effect of circulating catecholamine’s compared with EMT and NET.
📺 Watch the vodcast on noradrenergic transmission, including synthesis, release, postsynaptic effects, and breakdown of NE (5:32 min).
Sympathomimetic Drugs
There are a number of drugs that act upon the sympathetic nervous system. The direct acting adrenoceptor agonists include:
- adrenaline
- noradrenaline
- dopamine
- dobutamine
- isoprenaline
- metaraminol
- pseudoephedrine.
Some of these drugs will be discussed in some detail in this module. There are a number of drugs that are specific agonists at the β2 receptor (for example, salbutamol, terbutaline and salmeterol). These drugs will be covered in a subsequent module that deals with drugs that affect the respiratory system. Drugs that specifically block the alpha and beta receptors include the drug class Beta-blockers (for example atenolol, carvedilol, metoprolol and propranolol) and the drug class alpha-blockers which include clonidine, methyldopa, moxonidine and prazosin. Drugs that block alpha and beta receptors will be covered in the subsequent module on drugs that affect the cardiovascular system. Let’s not worry too much about them for now. Instead we will concentrate on those drugs we would class as sympathomimetics. Those exogenous drugs that are designed to mimic the endogenous catecholamines (adrenaline and noradrenaline)
The sympathomimetics (sometimes also referred to as catecholamines) are clinically used to treat a range of acute cardiovascular presentations including acute decompensating heart failure, cardiac arrest, profound hypotension and arrhythmias. They are also used to treat anaphylactic reactions and bronchospasm. This group includes dopamine, noradrenaline, adrenaline, isoprenaline and dobutamine. It is useful to treat this group as a class and then consider the differences and it is also useful to have an overview of the effect of catecholamines at alpha and beta receptors.
The sympathomimetic drugs mimic the effect of endogenous catecholamines (adrenaline, noradrenaline and dopamine) at the adrenoreceptors causing a range of responses depending on the sympathomimetic, dose and their affinity for the specific receptor. The physiological response may include increased heart rate and contractility, vasoconstriction of arterioles and veins, reduction of afterload via vasodilatation and bronchodilation but have a range of other effects on many organs.
| Sympathomimetic | Receptor activity | Physiological effect | ||||||
| Drug | Dose | Alpha | Beta1 | Beta2 | VD | VC | INT | CHT |
| adrenaline (epinephrine) | low
high |
+
+++ |
+++
++ |
++
– |
++
_ |
–
+++ |
+++
++ |
++
++ |
| dobutamine | + | +++ | + | ++ | – | +++ | + | |
| dopamine | low
intermediate high |
–
+ +++ |
++
++ ++ |
+
+ – |
++
+ – |
–
++ +++ |
–
++ ++ |
–
++ ++ |
| isoprenaline | – | ++++ | +++ | +++ | – | ++ | +++ | |
| noradrenaline (norepinephrine) | ++++ | + | – | – | ++++ | + | + |
VD=vasodilation; VC=vasocontriction; INT=positive inotropism; CHT=positive chronotropism; +=agonistic effect; -=no agonistic effect
🔑 Key concept:
Adrenaline:
Adrenaline is an exogenous and endogenous sympathomimetic that exerts much of its clinical effect through action at the β-adrenoreceptors in the heart, smooth muscle of the bronchioles and blood vessels. As the dose increases, it also demonstrates greater α-adrenoreceptor effect. At normal doses, adrenaline produces a positive inotropic effect (increased contractility of myocardial tissue) and a positive chronotropic effect (increased heart rate). It achieves a positive ionotropic effect as it increases the influx of calcium into the myocardial cells. The positive chronotropic effect results from a raising of the threshold action potential – this means the action potential threshold is achieved sooner and therefore more often… therefore heart rate can increase. This also partially explains the increased dromotropic effect (improvement in conduction of myocardial tissue). The net result is the increase in contraction achieves a more complete emptying of the ventricles. This coupled with an increased heart rate increases cardiac output (remember CO = SV x HR)!

- Adrenaline can either vasoconstrict or vasodilate depending on the vascular tissue involved. In large doses, α-adrenoreceptors are activated causing vasoconstriction, elevation in total peripheral vascular resistance and increases in blood pressure.
- Adrenaline also relaxes the smooth muscle of the bronchioles limiting the respiratory effects of allergens and also stabilizes mast cells to reduce the release of histamine (a pro inflammatory mediator common in allergic reactions).
The downside to adrenaline’s action is it also increases oxygen demands of myocardial tissue limiting its use in heart failure. Other side effects of adrenaline include headache, anxiety, palpitations, tachycardia, restlessness, tremor, hyperglycemia, elevation of blood pressure (plus more – think about your physiological response to a fright or threat and how your body responded). Where adrenaline is injected into peripheral tissues (like fingers, toes noses and ears), it can significantly constrict the blood supply and in extreme cases cause ischemia and necrosis distal to the site.
- Circulating adrenaline is rapidly metabolized by COMT and taken up into extra-neuronal tissue (for example – the liver) by ENT. The half-life of adrenaline is in the order of 2 minutes. Adrenaline is often administered by IV or IM injection but can also be given by nebulizer or by endotracheal tube.
📺 Watch the vodcast on adrenaline (8:02min).
Noradrenaline:
Noradrenaline is typically used to correct acute hypotension. It can increase both systolic and diastolic pressure because it tightens down on perfusion of arteriolar vascular beds (that is the vascular bed constricts) and has a weak effect at the β1-receptors causing some positive inotropic and chronotropic effect. It has a very high affinity for α-adrenoreceptors (found in the skin, visceral organs, mucous membranes and kidneys) and causes functional vasoconstriction. This results in decreased perfusion to these tissues and an elevation of the central blood pressure. The degree to which noradrenaline stimulates either α-adrenoreceptors or β1-adrenoreceptors is dose dependent. At low doses, β1-receptors are preferentially stimulated, at higher doses – α-receptor stimulation increases. The net clinical effect is to increase peripheral vascular resistance (mediated by α-receptors) and correct acute hypotensive states.

Because noradrenaline is rapidly metabolised by CMOT and MAO in the GIT, liver and other tissues, oral administration is of little value (this is really applicable to all of the sympathomimetic drugs discussed in this section except of pseudoephedrine). Reduction of circulating levels of noradrenaline is mostly achieved by uptake by NET into sympathetic neuronal tissues. Noradrenaline is often administered via a central venous line (similar to an IV line except the drug is administered directly into one of the larger veins. As the half-life is also very short (30-180 seconds) is it typically administered as a continuous infusion. Adverse reactions and drug interactions are similar to that of adrenaline – anxiety, tremor, insomnia, headache, palpitations, hypertensions and sometimes bradycardia (a compensatory response to elevated blood pressure).
📺 Watch the vodcast on noradrenaline (3:17 min).
Dobutamine:
Dobutamine is a synthetic catecholamine used to manage acute decompensating heart failure, septic shock and cardiogenic shock. It has the most significant β1 receptor (agonist) activity with some action at the α and β2 receptors. It increases cardiac output by enhancing stroke volume rather than increasing heart rate significantly – that is it increases the force of contraction and not so much the heart rate. Because it has comparatively less effect at the α-receptors, it does not cause constriction of the arteriovascular bed and therefore limits increases in peripheral vascular resistance. It is therefore useful in patients with low cardiac output as it can increase cardiac output (via increased inotropic effect) without significant increases in heart rate or blood pressure – two predictors of increase cardiac oxygen demand. Dobutamine like the other sympathomimetics are administered via an IV infusion and its side effects are similar to those of adrenaline.
📺 Watch the vodcast on dobutamine (4:47min).
Dopamine:
Dopamine, like noradrenaline, increases perfusion to vital organs and is used to improve the perfusion of the vital organs in circulatory shock or severe heart failure. Dopamine is an agonist at D1, α1, α2, and β1 receptors but has different effects depending on the administered dose. At low doses, dopamine interacts with the D1 (Dopamine subtype 1 receptor) causing renal artery dilatation with the effect of increasing blood flow to the kidneys (and increasing urine production and sodium excretion). At low to moderate does, dopamine acts directly on β1 receptors increasing myocardial contraction and stroke volume, increasing cardiac output. Like dobutamine, at moderate doses, peripheral vascular resistance is not greatly increased. Once higher doses are administered, the α-adrenoreceptors are stimulated increasing peripheral vascular resistance. Dopamine has a fairly rapid onset of action of between 2-5 minutes and an active duration of 5-10 minutes. Therefore, like the other catecholamines, it is administered by continuous infusion typically via a large central vein. Dopamine is metabolised by COMT and MAO. Dopamine does not readily cross the blood brain barrier so does not have clinically relevant central nervous system effects.
📺 Watch the vodcast on dopamine (2:14 min).
Isoprenaline:
Isoprenaline is a non-selective β-adrenoreceptor agonist mainly used to treat serious episodes of heart block. Its activity at β1 receptors increases myocardial contractility and heart rate and it also improves atrioventricular nodal conduction. The net effect is an increase in cardiac output and an increase in venous return to the heart.
📺 Watch the vodcast on Isoprenaline (0:56min).
Pseudoephedrine and Phenylephrine:
Pseudoephedrine and Phenylephrine, are partial agonists (weekly activates) of both α and β receptors. Its main clinical use is as a decongestant where they stimulate the α receptors in the nasal mucosa causing a vasoconstriction of the dilated blood vessels that supply the nasal mucosa resulting in a drying of nasal secretions.
Both are administered in an oral formulation (although there are topical sprays (oxymetazoline and xylometazoline) and can muscle tremor, palpitations, bradycardia, tachycardia, hypotension, hypertension, nausea and vomiting.
📺 Watch the vodcast on pseudoephedrine and phenylephrine (1:18 min).
Summary
- Adrenaline and noradrenaline are the two main neurotransmitters in the SNS
- The primary receptors in the SNS are α1, α2, β1 and β2
- Release of sympathetic neurotransmitter has a negative feedback effect on the release of the neurotransmitter
- neurotransmitter reuptake is the primary way of inactivating sympathetic neurotransmitters – NET and ENT are the main two transporters
- MAO and COMT are other important metabolic enzymes in the inactivation of sympathetic response
- Sympathomimetic agents in common use include adrenaline, noradrenaline, dopamine, dobutamine and isoprenaline
- Their different effects on the receptors account for differences in clinical response and use
- The duration of action of the sympathomimetic agents is short and this affects the administration route
- Drugs within the class share side effects.
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING
This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act.
Do not remove this notice.
