10.1 Intro to Pain and Pain Pathways
Pharmacology of Drugs Used for Pain
In this topic, we will look at the pharmacology of non-steroid anti-inflammatory drugs (NSAIDs). We will learn about the two classes of NSAIDs and explore the differences in their pharmacological effects throughout the body. We will also look at the pharmacology of paracetamol, its metabolism and the dangers of overdose.
Learning Outcomes
By the end of this module, it is expected students should be able to demonstrate and apply knowledge of:
- LO1 describe the mediators, receptors and channels involved in nociception, and the neural pathways for ascending transmission and descending inhibition of pain (linked to NEURO)
- LO2 identify the sites of action of analgesic drugs in these processes and pathways
- LO3 describe the cellular mechanisms responsible for the therapeutic effects of NSAIDs
- LO4 describe the main adverse effects of NSAIDs and relate these to their target enzymes
- LO5 describe the proposed mechanisms of action of paracetamol
- LO6 describe the cellular mechanisms of action of opioids in analgesia
- LO7 outline the mechanisms responsible for the major adverse effects of opioids
- LO8 describe the mechanisms of action of tricyclic antidepressants and anticonvulsants in the treatment of neuropathic pain
- LO9 outline the clinical use of analgesic classes in the treatment of nociceptive vs neuropathic pain
- LO10 state the mechanism of action of local anaesthetics including the concept of use-dependence
- LO11 explain the effect of pH on the action of local anaesthetics.
Intro to Pain and Pain Pathways
We have all experienced pain. Pain serves an important physiological role, it alters us to actual or potential injury however pain can also become disordered.
The International Association for the Study of Pain (IASP) define pain as: “An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage”.
In 2020, IASP provided six additional notes regarding pain:
- Pain is always a personal experience that is influenced to varying degrees by biological, psychological, and social factors.
- Pain and nociception are different phenomena. Pain cannot be inferred solely from activity in sensory neurons.
- Through their life experiences, individuals learn the concept of pain.
- A person’s report of an experience as pain should be respected.
- Although pain usually serves an adaptive role, it may have adverse effects on function and social and psychological well-being.
- Verbal description is only one of several behaviours to express pain; inability to communicate does not negate the possibility that a human or a nonhuman animal experiences pain.
Whilst acute pain can is important to alert us of actual or potential injury, chronic pain (>3months) can lead to significant morbidity and a very high burden on the healthcare system. Chronic pain is responsible for 40% of all early retirements and almost half of chronic pain sufferers also experience depression or anxiety.
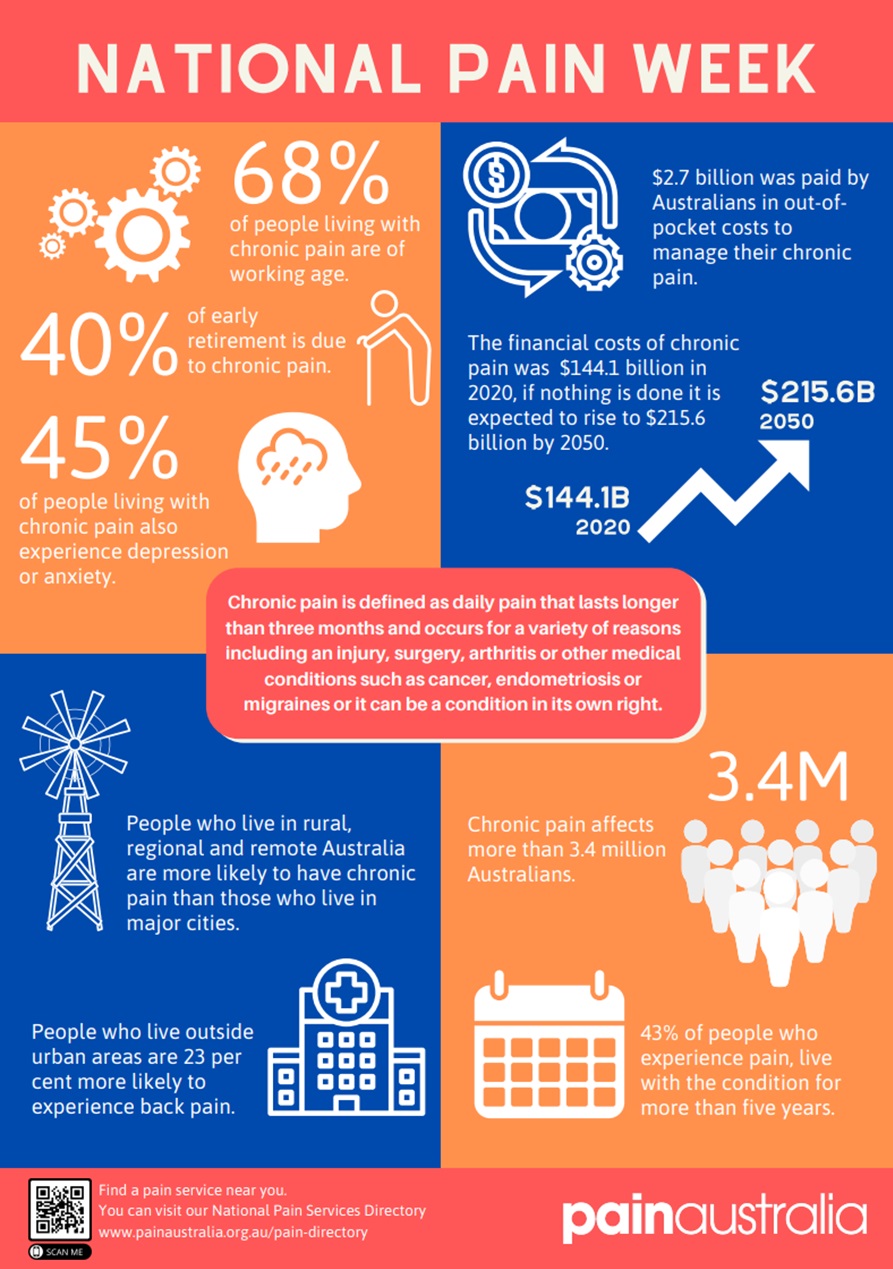
Although this module will discuss the pharmacology of analgesics, it is vital to acknowledge that analgesics are associated with very real risk of harm. Pain (especially chronic pain) is best managed by a multidisciplinary team (where available).

Pain Pathways (as they relate to pharmacology)
Before we begin to discuss the pharmacology of analgesics, it is important to understand the generation, transmission, and regulation of nociception (perception of noxious stimuli). You will cover this in greater detail within the NEURO component but there are important concepts that will help you to understand the pharmacology. It is also important to acknowledge that our understanding of the pharmacology of analgesics may at times lean on an incomplete comprehension/application of all elements of nociception.
Watch this YouTube video for a quick reminder of pain transmission and regulation
Please note that the video above does not provide a complete explanation of all anatomical features involved in the pain pathway, nor does is discuss all the chemical mediators involved in pain signalling. More complete information will be provided in NEURO and additional chemical mediators will be introduced in this module.
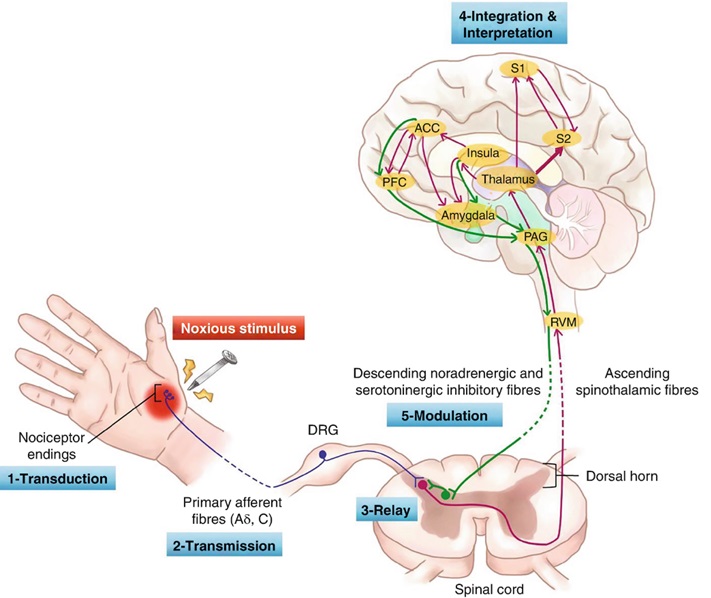
Ascending pain pathway
The ascending pain pathways are responsible for the detection of noxious stimuli and transmission of these signals to the somatosensory cortex for the perception of pain.
1st Order Nociceptive Neurons
Nociceptors are afferent neurons (1st order neurons) with their sensory region located in peripheral tissues. The presence of various stimuli facilitates depolarisation which triggers an action potential.
The action potential propagates along the neuron ultimately progressing to the dorsal horn of the spine where the neuron ends in a presynaptic terminal that releases the neurotransmitter glutamate (and possibly ATP).
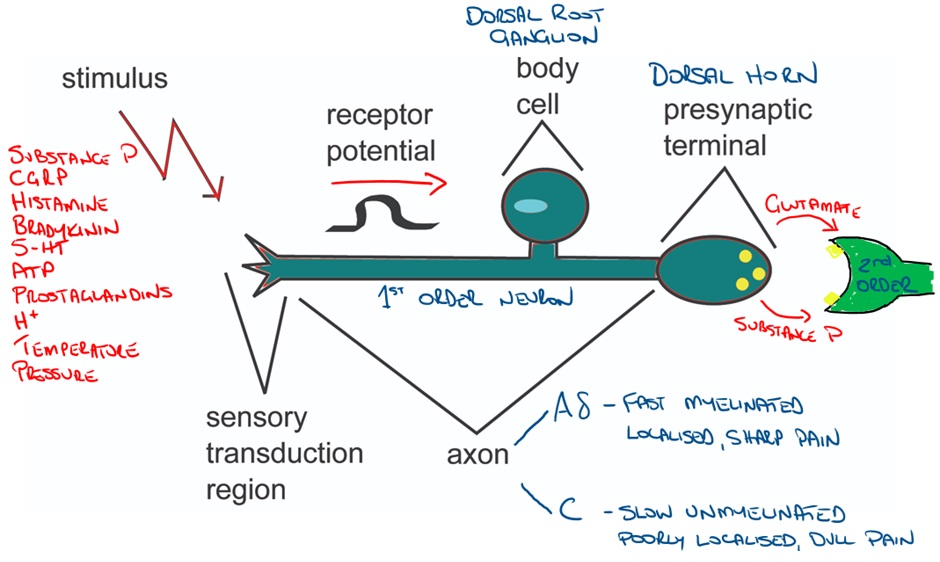
Image adapted from: Nociception – Physiopedia (physio-pedia.com)
Additional neuropeptides including substance P, calcitonin gene-related peptide (CGRP) and galanin also play important roles in nociception.
Substance P and CGRP act peripherally to promote inflammation (neurogenic inflammation) which leads to the production of additional ‘noxious’ chemical stimulators while galanin produces anti-inflammatory effects. Within the dorsal horn, Substance P is thought to sensitise 2nd order neurons to glutamate (increasing transmission to higher centres) and appears to be most active in regions of the dorsal horn where C fibres terminate.
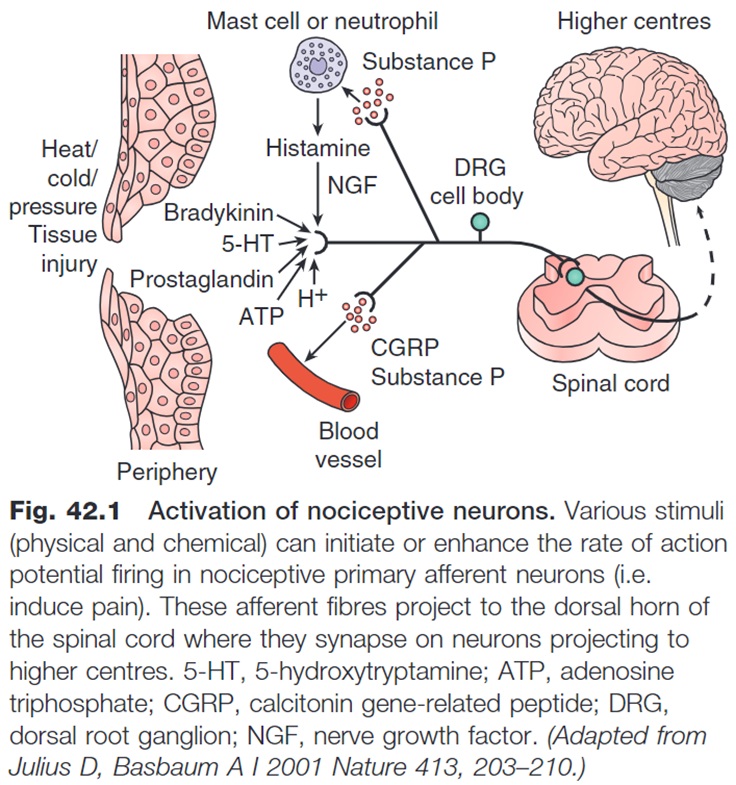
If we take a deeper look at the chemical mediators, receptors and channels present at the sensory end of the 1st order neuron, we can see the complex interplay of numerous chemical messengers, receptors and ion channels involved in triggering depolarisation and action potential generation within the 1st order neuron.
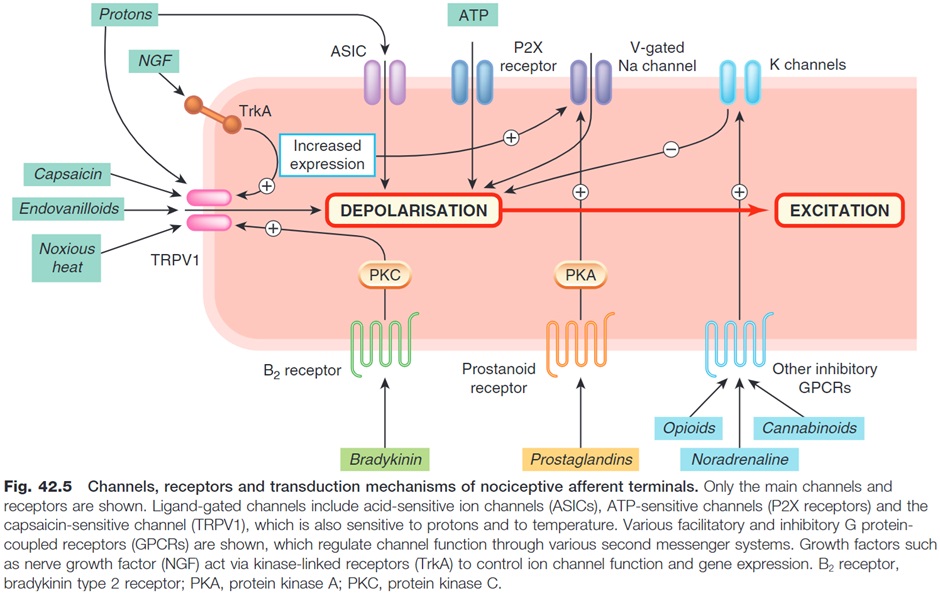
Non-Steroidal Anti-Inflammatories (NSAIDs) are a classic example of an analgesic that works to reduce chemical stimulation at the sensory end of the 1st order neuron.
Dorsal Horn
The 1st order neuron terminates within the dorsal horn where it synapses with the 2nd order neuron. The 2nd order neuron then proceeds to the thalamus via the contralateral spinothalamic tract.
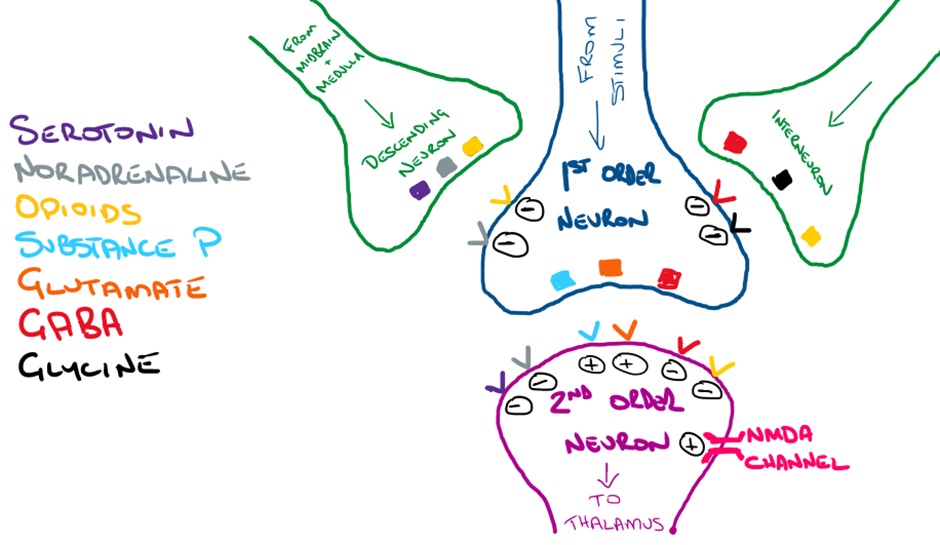
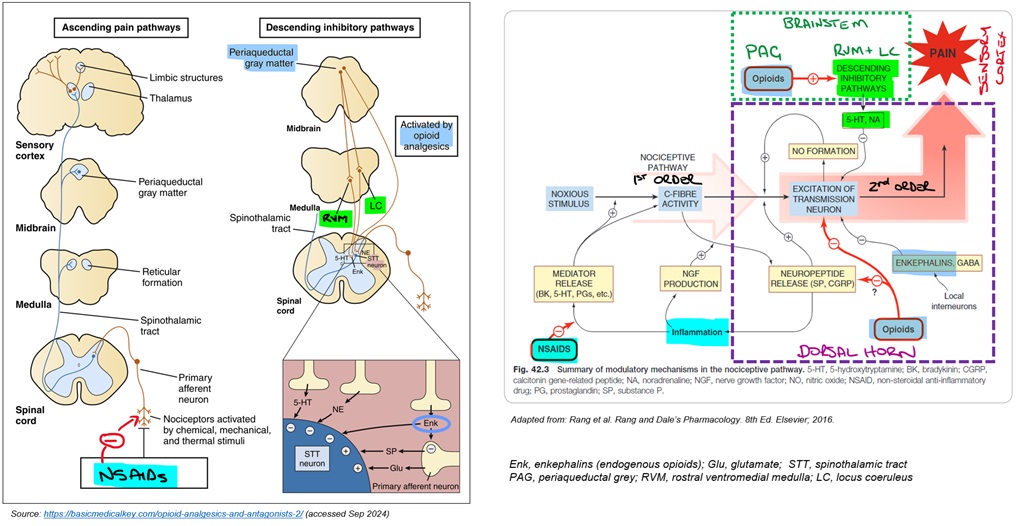
In terms of pharmacology, the modulation of pain within the dorsal horn is particularly important. Descending neurons and interneurons release inhibitory neurotransmitters (eg enkephalins) that reduce depolarisation of both presynaptic 1st order neurons and post-synaptic 2nd order neurons, reducing the transmission of nociceptive signals to the thalamus. These inhibitory processes are sometimes referred to as the ‘pain gate’.
Let’s take a closer look at some of the neurotransmitters and their receptors within the dorsal horn. In the image below, you can see that endogenous opioids, serotonin and noradrenaline are all potential targets to reduce pain transmission.
2nd order neurons transmit signals up the spinothalamic tract eventually terminating in the thalamus where 3rd order neurons then relay the signals to the somatosensory cortex. It is in the somatosensory cortex that the pain is perceived.
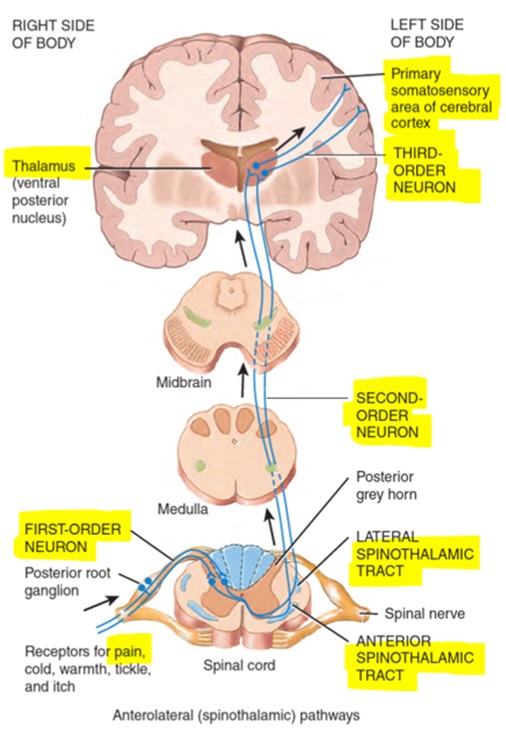
Source: Chapter 16.3 Somatic Sensory Pathways. In: Tortora et al. Principles of Anatomy and Physiology. First Asia-Pacific ed. Queensland, Australia: John Wiley & Sons; 2016.
Descending Pain Pathways (Inhibitory)
We’ve briefly discussed the ascending pain pathways, but they are only half the story. It is equally important (or perhaps more important for pharmacology) to understand the descending (modulating/inhibitory) pain pathways.
The descending pathways play an important role in modulating the pain transmission in the ascending pathways. From a pharmacology perspective, we will focus on the inhibitory effects of the descending pathways although the descending pathways are not exclusively inhibitory.
Descending pathways may originate in higher centres however much of our understanding focuses on the actions of inhibitory neurons that originate within regions of the brainstem.
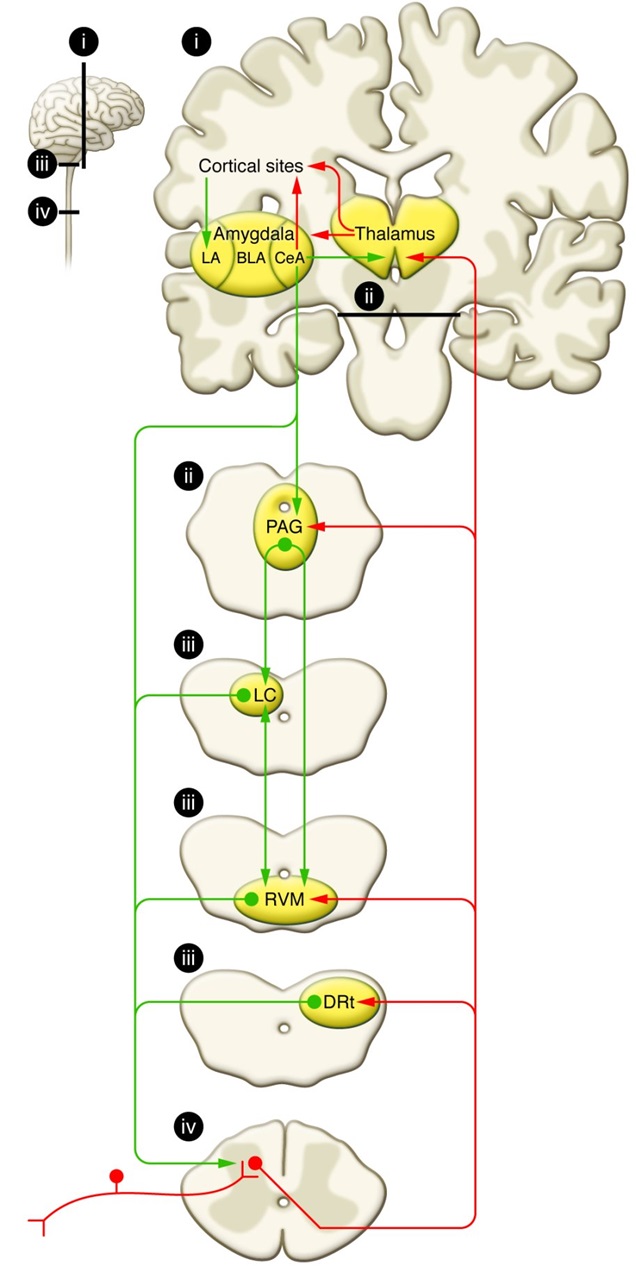
The periaqueductal grey (PAG) is located around the cerebral aqueduct within the midbrain. PAG neurons extend to the locus coeruleus (LC) in the pons and the raphe nuclei within the rostral ventromedial medulla (RVM). Neurons arising in the LC are noradrenergic while those arising in the raphe nuclei are serotonergic.
From here, these neurons descend to the dorsal horn where they have their inhibitory effects on propagation/relay of signals from 1st order to 2nd order neurons. Endogenous opioids (enkephalins) release from inhibitory neurons in the dorsal horn play an important role.
You will see a variety of naming conventions used in different resources and sometimes within the same resource that can be confusing.The dorsal horn is divided into laminae (I-VI). Laminae II is also known as the substantia gelatinosa and is particularly important when considering the action of opioids. The relay from 1st order to 2nd order neurons occurs in various laminae.
The serotonergic raphe nuclei is located within the rostral ventromedial medulla (RVM) and is sometimes referred to as the nucleus raphe magnus.
Some resources also refer to a triad within/adjacent to the reticular formation comprising the RVM, caudal ventrolateral medulla (VLM) and the subnucleus reticularis dorsalis (DRt). Perhaps most importantly, although they may be somewhat anatomically distinct their function in pain modulation is highly interdependent.
In this module, we will aim to simply things by referring to the following regions: dorsal horn, periaqueductal grey, locus coeruleus (LC) and rostral ventromedial medulla (RVM) with an understanding that this may not completely describe all anatomical or functional regions involved.
Hopefully this helps to avoid confusion…
Coming back to the pharmacology, the following diagram begins to pull together various elements. Each of these will be discussed in more detail as we move through the different analgesics in more detail.
🎥 Watch this video
Lecture slides:
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.