5.1 Haemostasis and Blood Coagulation Process
Introduction
This week we will focus on the main features of blood coagulation, platelet function and fibrinolysis. These processes underlie haemostasis and thrombosis and provide a basis for understanding haemorrhagic disorders (e.g. haemophilia) and thrombotic diseases of both arteries (e.g. thrombotic stroke, myocardial infarction) and veins (e.g. deep vein thrombosis, pulmonary embolism). Anticoagulants, antiplatelet drugs and fibrinolytic drugs are especially important because of the prevalence of thrombotic disease.
Haemostasis
Haemostasis is the process by which the body halts or reduces bleeding from damaged blood vessels, a crucial survival mechanism for organisms in potentially dangerous environments. When a wound occurs, the blood vessels constrict, initiating a cascade of events including:
- Platelet adhesion and activation
- Fibrin formation
Activated platelets create a haemostatic plug that stops the bleeding, which is then strengthened by fibrin. The significance of these processes varies depending on whether the injury occurs in an artery, vein, or capillary.
On the other hand, thrombosis refers to the abnormal formation of a haemostatic plug/clot within blood vessels without the presence of bleeding, essentially “haemostasis in the wrong place.” Over a century ago, Rudolph Virchow identified three factors that predispose individuals to thrombosis, known as “Virchow’s triad”: damage to the vessel wall (such as when an atheromatous plaque ruptures or erodes), altered blood flow (as seen in the left atrial appendage during atrial fibrillation or in leg veins during prolonged immobility), and increased blood coagulability (which may occur in the later stages of pregnancy or with certain oral contraceptive use). Inherited conditions leading to increased coagulability are termed thrombophilia.
It’s important to distinguish a thrombus, which forms within the body, from a clot that forms outside the body, such as in a test tube. Clots are amorphous with a loose fibrin network trapping blood cells, while thrombi have a more defined structure depending on their arterial or venous origin.
Arterial thrombi are often linked with atherosclerosis and consist mainly of platelets in a fibrin matrix, known as a white thrombus. These thrombi can disrupt blood flow, potentially leading to tissue damage or infarction downstream. Venous thrombi, on the other hand, consist of a small white head and a larger red tail, similar in composition to a blood clot, and are known as red thrombi. These thrombi can detach and travel through the bloodstream as emboli. Venous emboli frequently lodge in the pulmonary arteries, causing pulmonary embolism, whereas emboli from the left heart or carotid arteries may obstruct blood flow in the brain or other organs, leading to severe outcomes like stroke or death.
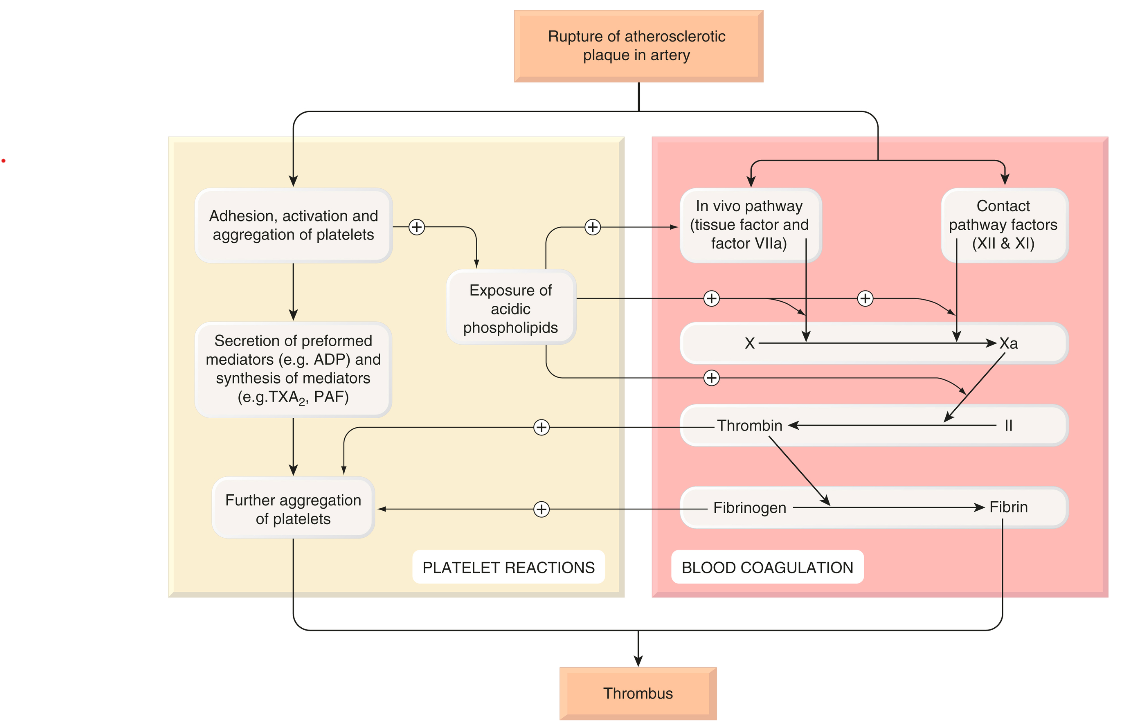
Drug therapy plays a key role in managing haemostasis and thrombosis. In cases where haemostasis is impaired—such as in haemophilia or after excessive anticoagulant use—medications that promote blood clotting, such as antifibrinolytic and haemostatic drugs, are necessary. On the other hand, treating or preventing thrombosis and thromboembolism is critical given the prevalence and severity of these conditions. Drugs used for these purposes can influence haemostasis and thrombosis through three main mechanisms: altering blood coagulation (fibrin formation), modifying platelet function, or enhancing fibrin removal (fibrinolysis).
- blood coagulation (fibrin formation)
- platelet function
- fibrin removal (fibrinolysis).
Blood Coagulation
Coagulation Cascade
Blood coagulation refers to the process where liquid blood transforms into a clot. This process primarily involves the conversion of soluble fibrinogen into insoluble fibrin strands, a reaction catalyzed by thrombin. This transformation represents the final step in a complex sequence of enzymatic reactions known as the coagulation cascade. The cascade involves various components, termed factors, which exist in the blood as inactive precursors (zymogens) of proteolytic enzymes and cofactors. These factors become activated through proteolysis, with their active forms designated by the suffix “a.” Key factors in this cascade, including XIIa, XIa, Xa, IXa, and thrombin (IIa), are serine proteases. A small amount of an activated factor can catalyze the production of a larger quantity of the next factor, thus amplifying the process in a cascading manner. However, this rapid amplification must be regulated to prevent excessive clotting, which could result in the solidification of blood throughout the body. Antithrombin III plays a crucial role in this regulation by neutralizing all serine proteases in the cascade. Additionally, the vascular endothelium actively prevents the extension of a thrombus.
Traditionally, two pathways for fibrin formation were identified: the intrinsic pathway (comprising components present within the blood) and the extrinsic pathway (involving components from outside the blood). The intrinsic, or “contact,” pathway is activated when blood comes into contact with artificial surfaces, like glass, but under physiological conditions, both pathways operate as a single, unified system in vivo. Tissue damage exposes blood to tissue factor, which initiates the coagulation process, leading to the production of a small amount of thrombin. This thrombin then acts through several positive feedback loops (on Va, VIIIa, and platelets) to amplify and propagate the process, resulting in the production of more thrombin.
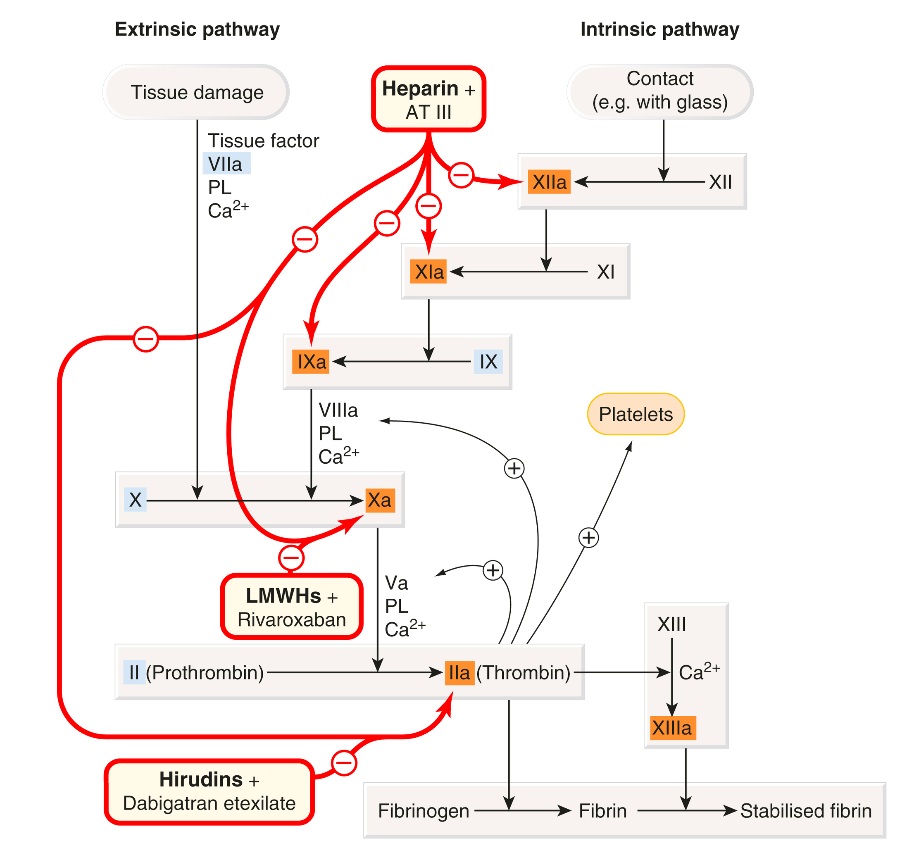
The cellular receptor for factor VII is known as ’tissue factor.’ When Ca²⁺ is present, this interaction triggers an active site transition, leading to the swift autocatalytic conversion of factor VII into its active form, VIIa. The tissue factor–VIIa complex subsequently activates factors IX and X. During platelet activation, acidic phospholipids (PLs), particularly phosphatidylserine, are exposed on the outer membranes of platelets, functioning as surface catalysts that facilitate the activation of various clotting factors by bringing them together in functional complexes. Platelets also secrete coagulation factors, such as factor Va and fibrinogen, contributing further to the clotting process. Sustained coagulation relies on the continued production of factor Xa through the IXa–VIIIa–Ca²⁺–PL complex, as the tissue factor–VIIa complex is quickly neutralized in plasma by tissue factor pathway inhibitor and antithrombin III. Factor Xa, in conjunction with Ca²⁺, PL, and factor Va, converts prothrombin into thrombin, which is the key enzyme in the coagulation cascade. The intrinsic (contact) pathway begins when factor XII (Hageman factor) attaches to a negatively charged surface, eventually merging with the in vivo pathway at the point of factor X activation. Although the initial part of this pathway is not essential for in vivo blood coagulation, both pathways are interconnected, and various positive feedback mechanisms further enhance the coagulation process.
Thrombin (factor IIa) enzymatically cleaves fibrinogen, producing fragments that polymerise to form fibrin. It also activates factor XIII, a fibrinoligase, which strengthens fibrin-to-fibrin links, thereby stabilising the coagulum. In addition to coagulation, thrombin also causes platelet aggregation, stimulates cell proliferation and modulates smooth muscle contraction. Paradoxically, it can inhibit as well as promote coagulation.
|
Blood coagulation (fibrin formation) – In brief The clotting system consists of a cascade of proteolytic enzymes and co-factors. • Inactive precursors are activated sequentially in an amplifying cascade. • The last enzyme, thrombin, derived from prothrombin (II), converts soluble fibrinogen (I) to an insoluble meshwork of fibrin in which blood cells are trapped, forming the clot. • There are two limbs in the cascade: − the in vivo (extrinsic) pathway − the contact (intrinsic) pathway • Both pathways result in activation of factor X to Xa, which converts prothrombin to thrombin. • Calcium ions and a negatively charged PL are essential for three steps, namely the actions of: − factor IXa on X − factor VIIa on X − factor Xa on II • PL is provided by activated platelets adhering to the damaged vessel. • Some factors promote coagulation by binding to PL and a serine protease factor; for example, factor Va in the activation of II by Xa, or VIIIa in the activation of X by IXa. • Blood coagulation is controlled by: − enzyme inhibitors (e.g. antithrombin III) − fibrinolysis.
|

Lecture slides
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNINGThis material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.
