6.1 Diabetes Introduction
Learning Outcomes
Be able to:
-
Describe the physiological role of insulin and the basic pathophysiology of type 1 and 2 diabetes mellitus. (REVIEW)
-
Describe the use of insulin therapy in type 1 and 2 diabetic patients.
-
Compare the different formulations of insulin and outline how they are used in daily regimens.
-
Describe the mechanisms of action, major adverse effects, and precautions associated with metformin, sulfonylureas, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 analogues, and sodium-glucose co-transporter 2 inhibitors.
-
Outline the general approach to drug therapy in patients with type 2 diabetes.
We will not be covering diabetes insipidus this week. Diabetes insipidus is related to dysregulation of arginine vasopressin (AVP), also known as antidiuretic hormone. It is not related to elevated blood glucose levels, nor is it managed with antihyperglycaemic agents.
What is diabetes Mellitus?
Diabetes has been described for millennia; Indian surgeon Sushruta described ‘honey like urine’ in the 5th century BC. The name diabetes comes from Greek for siphon and mellitus from Latin meaning honeyed or sweet and describes the presentation of polyuria and associated glucosuria.

Interesting history to one side, diabetes mellitus is a complex chronic condition underpinned by elevated blood glucose levels due to insulin deficiency (and/or resistance) and the subsequent clinical consequences.
Diabetes is divided into several types. Is it important to understand the differences between the types of diabetes and how this impacts treatment.
Type 1 diabetes (T1DM) or insulin dependent (IDDM)
- Insulin deficiency caused by auto-immune destruction of pancreatic beta cells that are responsible for insulin production.
- Non-modifiable, commonly presenting in childhood and adolescence.
- Treated with insulin.
- Approximately 10% of people with diabetes.
Type 2 diabetes (T2DM) or non-insulin dependent (NIDDM)
- Relative insulin deficiency and insulin resistance on a background of progressive loss of beta-cell function.
- Complex interplay of genetic and modifiable lifestyle factors typically presenting after 40yrs of age.
- Treated with lifestyle modifications, antihyperglycaemics and/or insulin.
- Approximately 85% of people with diabetes.
Gestational diabetes (GDM)
- Occurs during pregnancy and is associated with a higher risk of adverse pregnancy outcomes.
- Usually resolves following pregnancy however significantly elevated risk of T2DM development later in life.
- Treated with a combination of lifestyle and/or pharmacotherapy
- Approximately 4% of diabetics.
Other types of diabetes mellitus
- A variety of different comorbidities, medical treatments and genetic subtypes and environmental factors can also contribute to elevated blood glucose levels and insulin deficiency (either complete or relative).
- Managed similarly to T1DM or T2DM depending on underlying pathophysiology.
- Approximately 1% of diabetics.
Check your understanding:
How common is diabetes?
Approximately 1 in 20 Australians are currently living with diabetes, making it a common disease that is frequently encountered in clinical practice. Approximately 1.3 million hospitalisations occur each year due to diabetes and it is estimated that diabetes costs approximately $17.6 billion per annum in Australia.
The number of people living with diabetes in Australia has approximately doubled over the past 20 years. Fortunately, there appears to be a plateauing and possibly a small decrease in prevalence over recent years.
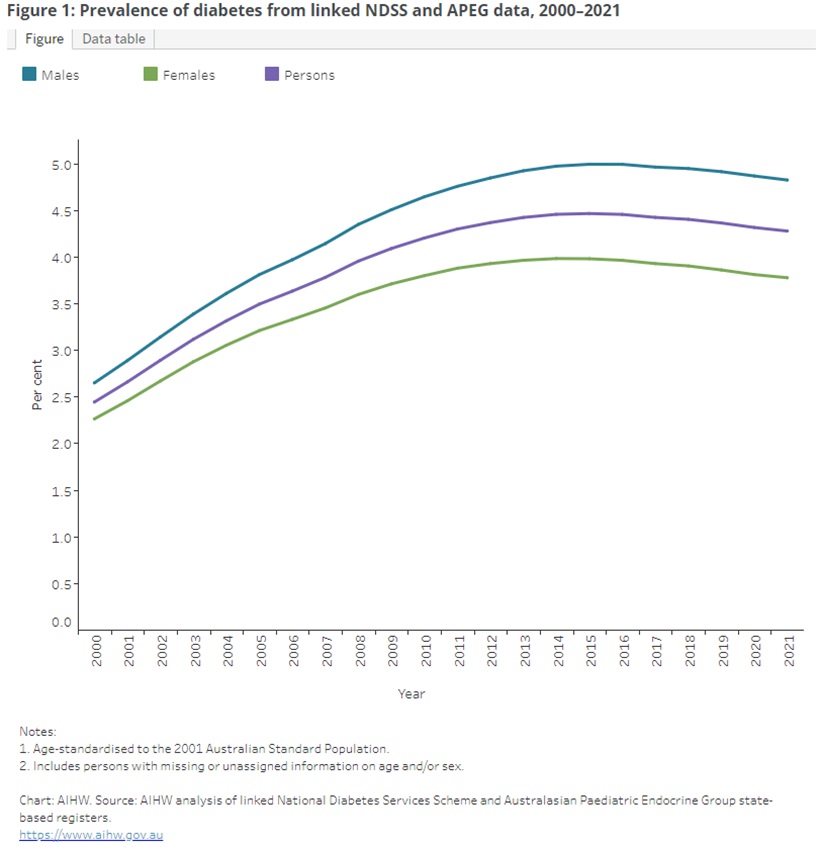
Like many other chronic conditions, the prevalence of T2DM increases with age. This is an important consideration as most patients will have multiple factors contributing to cardiometabolic dysfunction and cardiovascular risk which can influence the choice of therapy.
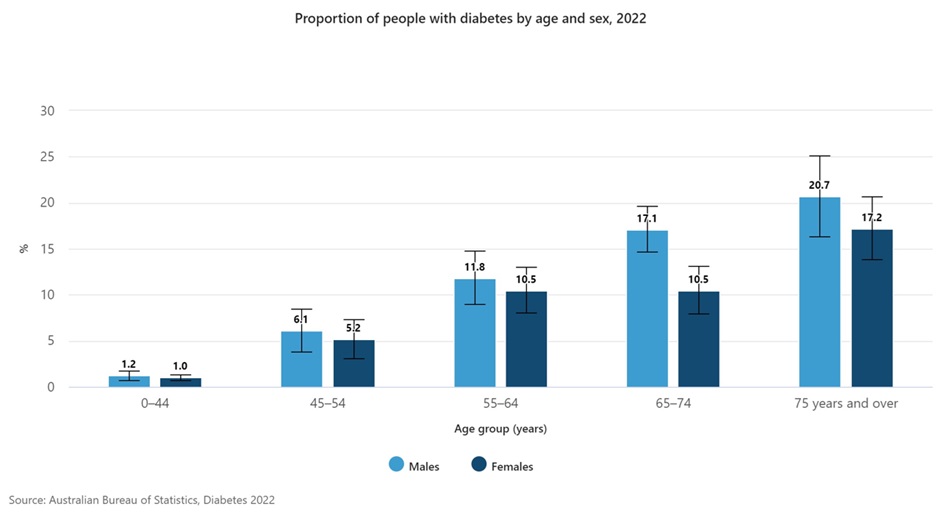
Over 19 million PBS prescriptions were dispensed in 2022-23 for diabetes therapies making them the 7th most dispensed (and prescribed) group of medications in Australia. A good understanding of these medications is very important as you will see them every day of practice.
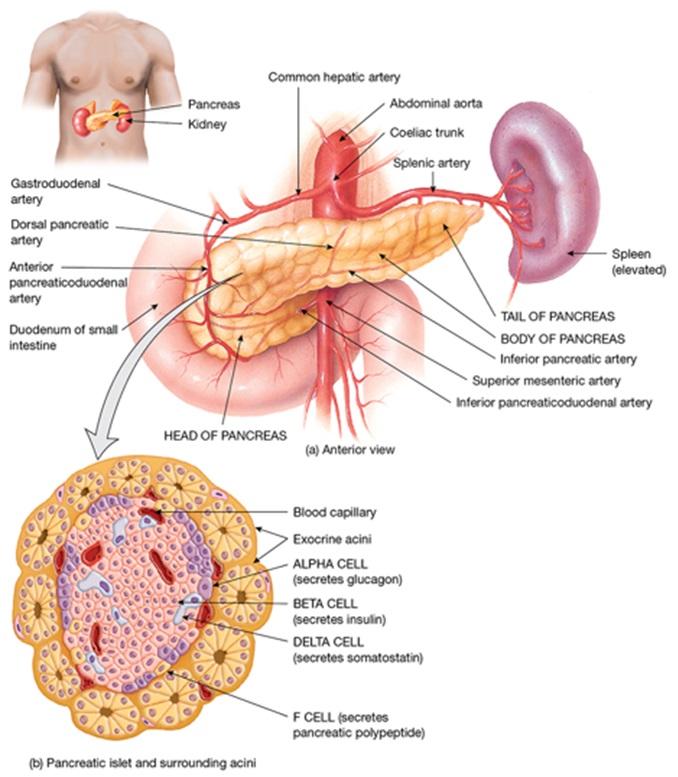
The endocrine pancreas
The pancreas performs a variety of exocrine and endocrine functions. This week we will be focussing on the endocrine functions of insulin (and glucagon) which are produced within the pancreas.
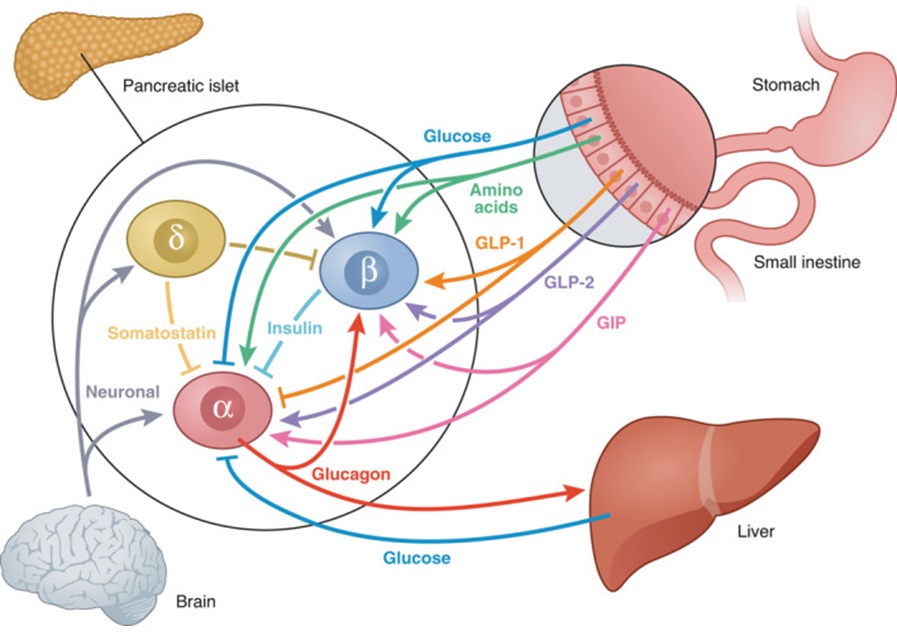
Insulin and glucagon are both peptide hormones produced by cells found in the islets of Langerhans. Insulin is produced in the beta cells while glucagon is produced in the alpha cells. Insulin is an anabolic (building) hormone while glucagon is a catabolic (breaking down) hormone.
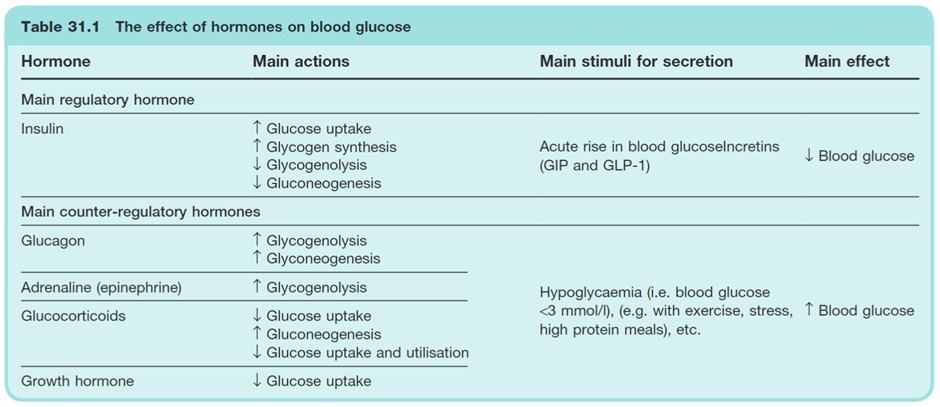
Insulin and glucagon have opposing effects on blood glucose levels and are released in response to differing physiological states. Insulin LOWERS blood glucose in the fed state while glucagon RAISES blood glucose levels in the fasting state.
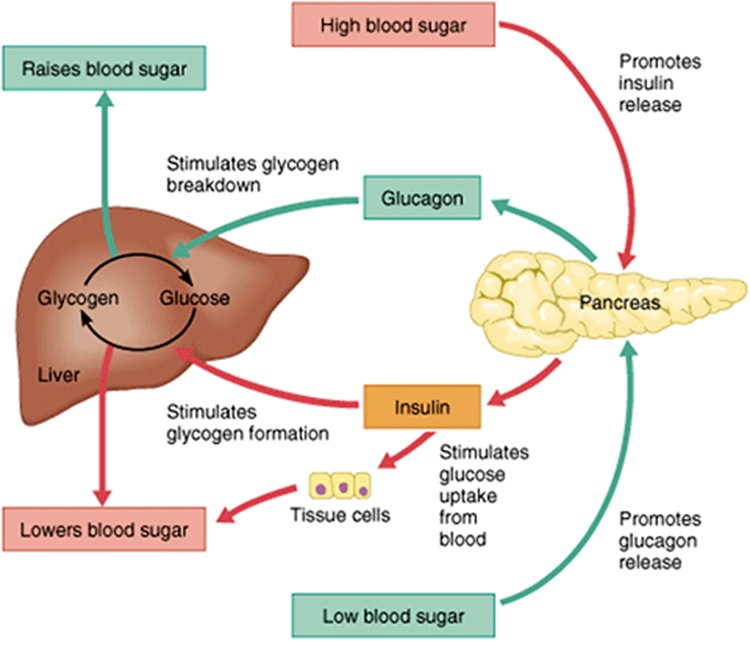
There is a close relationship between insulin, glucagon and other counterregulatory pathways that work to maintain ‘normal’ blood glucose levels and a constant supply of energy to cells throughout the day. A disruption in insulin leads to dysfunction throughout the associated pathways. The image below shows the interconnectedness of feedback loops at play in BGL regulation.
Insulin
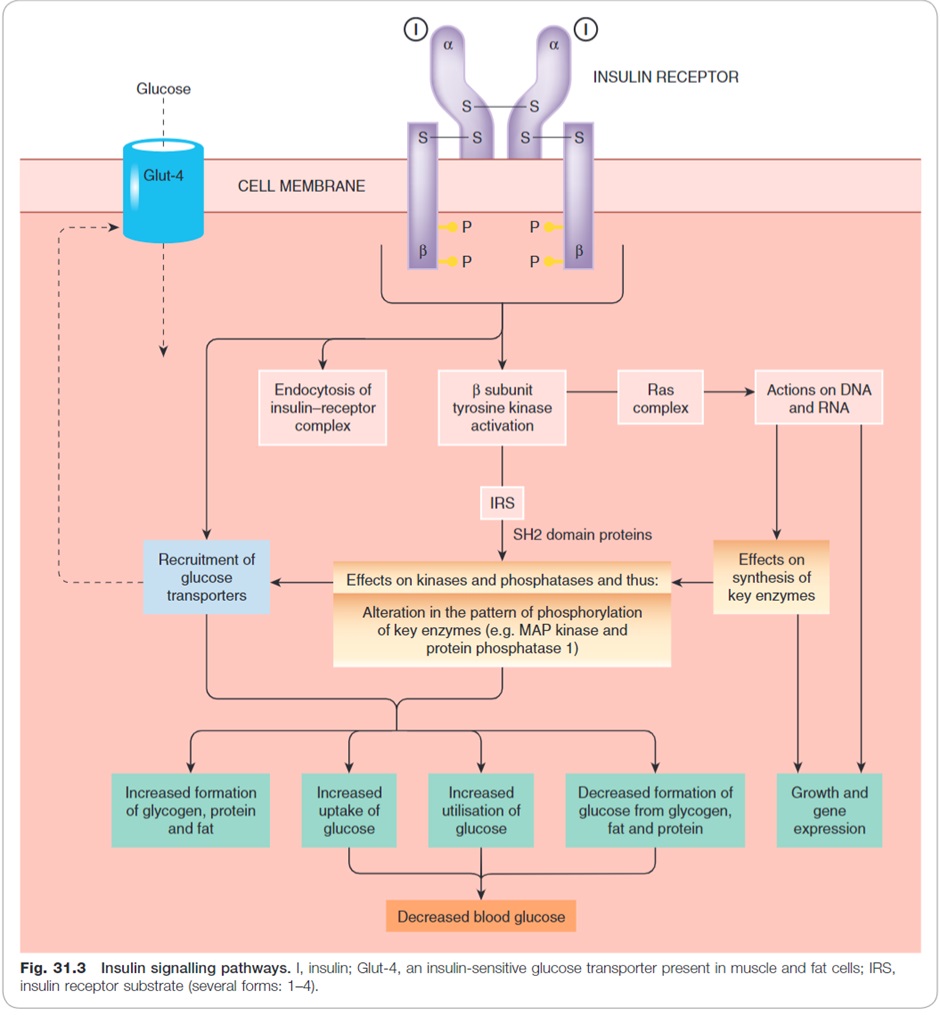
Insulin is released in the fed state in response to elevated blood glucose levels. When insulin binds to the insulin receptor (a tyrosine kinase receptor) on the cell membrane it triggers a variety of important cellular processes including:
- Increased expression of glucose transporters (GLUT4) on the cell wall which allow glucose to move across the cell membrane into muscle and adipose cells.
- Increase energy storage:
- Increased glycogen synthesis (glycogenesis)
- Fat synthesis (lipogenesis)
- Inhibit lipid and protein breakdown (lipolysis and proteolysis)
- Inhibit ketogenesis:
- Inhibit free fatty acid formation (occurs during lipolysis)
- Prevent free fatty acids entering mitochondria where they can be metabolised to ketoacids
- Inhibit glucagon production in alpha cells
Watch this YouTube video to help review insulin.
Glucagon
It is not possible to discuss insulin without also discussing glucagon. Glucagon is released from alpha cells in response to hypoglycaemia.
In the clinical context, an understanding of glucagon’s actions is vital to understanding the management of hypoglycaemia and why diabetic ketoacidosis occurs.
Hypoglycaemia:
- Glucagon triggers hepatic glucose production by converting glycogen stores into glucose (glycogenolysis). This hepatic glucose production leads to increased BGLs, correcting the hypoglycaemia.
Hyperglycaemia:
- Elevated blood glucose levels and subsequent insulin release from beta cells reduce glucagon release from alpha cells. Reduced glucagon release leads to lower hepatic glucose production, helping to prevent further elevation of BGL.
Diabetic ketoacidosis:
- In diabetes, there is a lack of insulin. The lack of insulin limits the negative feedback on glucagon release that normally occurs. The ‘unopposed’ glucagon release from alpha cells is therefore able to drive catabolic processes including lipolysis which produces free fatty acids that can ultimately be converted into keto acids.
Watch this YouTube video to help review glucagon.
Glucagon is only one of several counterregulatory hormones. Somatostatin, adrenaline, cortisol and growth hormone (amongst others) also play an important role in blood glucose regulation. To allow time to focus on the pharmacology of diabetes, these are not discussed in detail in this week.
Check Your Understanding:
But why do high BGLs matter?
When we consider the impact of diabetes, we need to consider both the short and long-term consequences of diabetes and the type of diabetes (T1DM vs T2DM).
Two particularly important acute complications include:
- Diabetic ketoacidosis (DKA) – most commonly occurs in people living with T1DM (there is no/little insulin to prevent keto acid formation). In Queensland, approximately 45% of children receive their T1DM diagnosis following a presentation with DKA. DKA is uncommon in T2DM but can occur and requires extra caution in people taking SGLT-2 Inhibitors (discussed later).
- Hyperosmolar hyperglycaemia (HHS) – most commonly occurs in T2DM. It is more common in elderly patients however some adults and teenagers with early onset T2DM may present with HHS at the time of diagnosis.
Both DKA and HHS are medical emergencies and present with dehydration and electrolyte abnormalities in addition to BGL elevations (normally). The dehydration and electrolyte abnormalities are primarily secondary to renal losses associated with polyuria and nausea/vomiting limiting intake. Thinking back to diuretics from week 2, you can see how elevated BGLs lead to an osmotic diuresis.
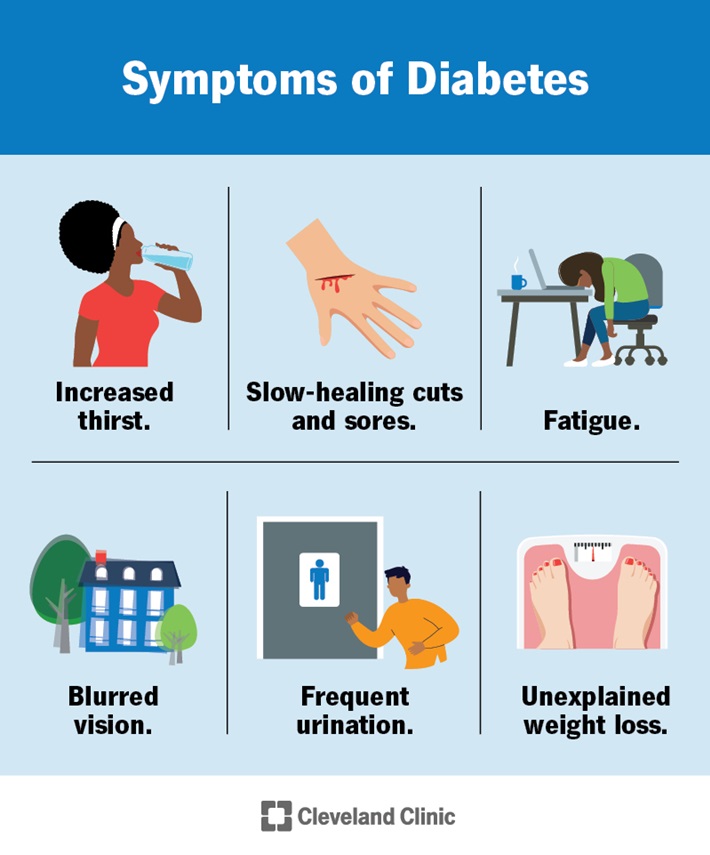
The classic symptoms of elevated BGLs that can occur with both T1DM and T2DM include:
- Excessive thirst (polydipsia)and frequent urination (polyuria)
- Lack of concentration and lethargy
- Blurred vision
- Change in behaviour (typically irritability)
Elevated BGLs are associated with:
- Direct glucose mediated endothelial damage
- Oxidative stress
- Advanced glycation end products
- Inflammation and inappropriate growth factor production
These processes lead to many of the significant vascular, renal and ophthalmic complications of poorly controlled diabetes.
You don’t need to understand the images below in detail but they provide a nice overview of mechanisms of glucose mediated damage in different organs.
Image sequence – click to slide across
“But I feel fine”
The objectives of diabetes management include:
- Optimise quality of life
- Relieve symptoms of hyperglycaemia
- Avoid acute complications of hyperglycaemia, such as diabetic ketoacidosis and hyperosmolar hyperglycaemia
- Avoid hypoglycaemia
- Reduce chronic complications of hyperglycaemia.
For an asymptomatic patient, we are usually most concerned with reducing their risk of developing chronic complications of diabetes. Pharmacotherapy plays an important role in achieving this goal in conjunction with non-pharmacological measures.
Much emphasis is placed on reducing the microvascular and macrovascular complications of diabetes and the associated morbidity and mortality:
- Microvascular complications
- Diabetic kidney disease
- Diabetic retinopathy
- Diabetic neuropathy
- Macrovascular complications
- Ischaemic heart disease
- Cerebrovascular disease
- Peripheral vascular (arterial) disease.
There are numerous chronic complications of elevated BGLs that impact a variety of body systems. These will not all be covered in detail this week but the image below provides a snapshot.
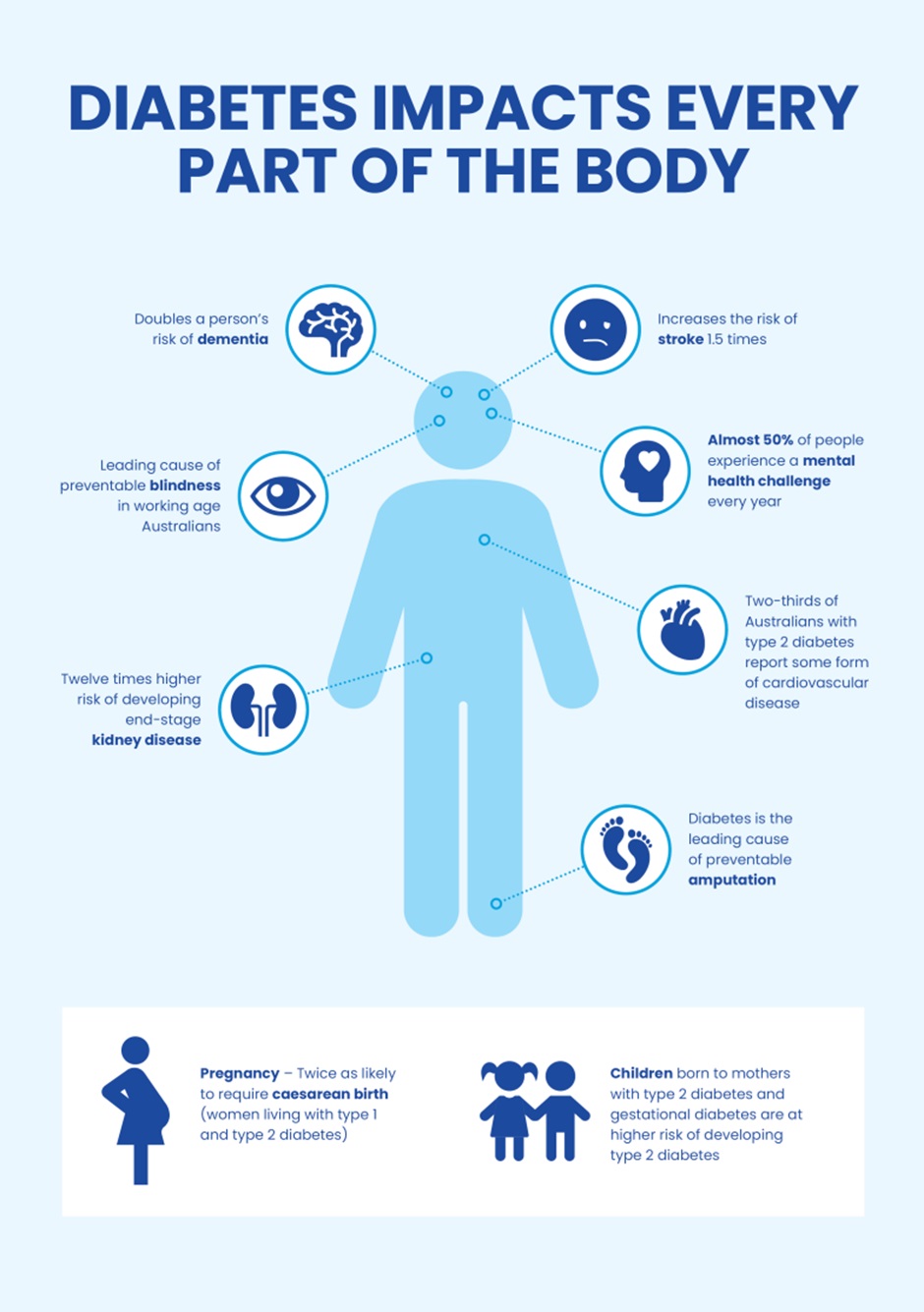
Check Your Understanding:
Watch this video – Overview of Diabetes and Insulin
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNINGThis material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.
