6.2 Insulin Use in Diabetes
Insulin Use in Diabetes
You will recall that in T1DM there is a total (or nearly total) insulin deficiency. As such, administration of exogenous insulin is the cornerstone of BGL management (and ketosis prevention) in T1DM. T1DM is also referred to as insulin-dependent diabetes mellitus (IDDM). Patients with T1DM require lifelong exogenous insulin.
In contrast, T2DM has residual insulin production and therefore insulin is typically only added when other therapies fail to provide sufficient BGL control or are contraindicated.
Insulin is a peptide hormone and susceptible to degradation in the gastrointestinal tract. Insulin is administered parenterally by subcutaneous (usually) or intravenous (infrequently) administration to avoid degradation in the gastrointestinal tract. If insulin intended for subcutaneous administration is inadvertently administered intramuscularly it typically leads to much faster onset of action and a higher risk of hypoglycaemia.
Insulin binding to insulin receptors results in increased expression of glucose transporters (GLUT4) on the cell wall which allows glucose to move across the cell membrane into muscle and adipose cells. This intracellular shift leads to significant BGL reduction. In the liver, insulin leads to a reduction in glycogenolysis, reduction in gluconeogenesis and increases glycogenesis resulting in a net reduction of BGL by reducing hepatic glucose release and increasing glucose conversion to stored glycogen.
Hypoglycaemia
Insulin’s potency also results in the highest risk of hypoglycaemia. The risk of hypoglycaemia is impacted by a variety of factors including insufficient food intake, exercise, alcohol consumption, illness, type of insulin, administration site and technique amongst numerous other factors.
Read/explore:
Patients using insulin require frequent BGL monitoring, and adjustment of their insulin dose based on BGL results and carbohydrate intake. Equipping patients and carers with the skills required to self-manage their insulin dose is very important.
Other side effects of insulin use
Hypoglycaemia is the most concerning side effect of exogenous insulin use. Insulin use is also associated with weight gain (remember insulin facilitates lipogenesis and resists catabolism) and injection site reactions.
Types of insulin and insulin devices
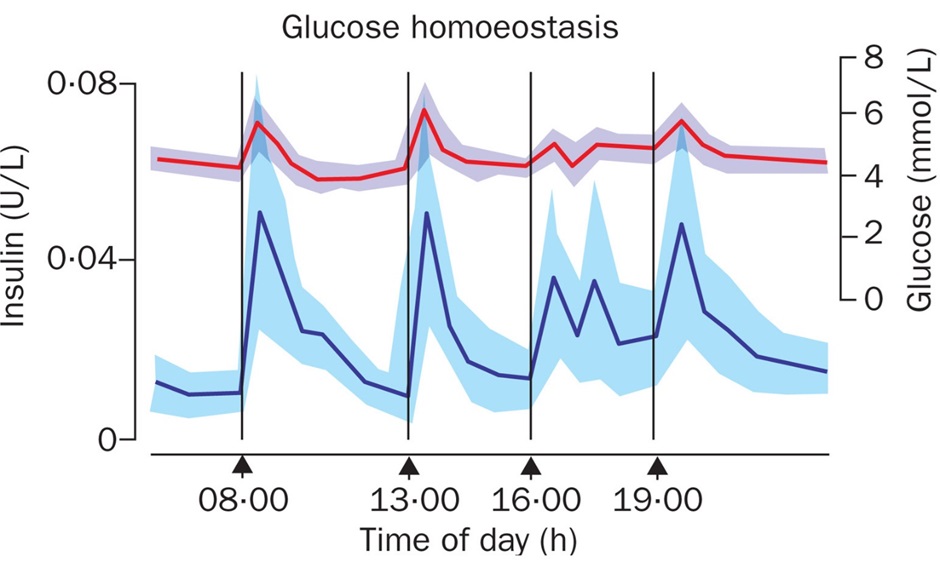
As you can see above, insulin release consists of two portions, a background ‘basal’ release and a prandial ‘bolus’ release in response to eating and subsequent increase in BGL, amino acids and incretins (we’ll learn about these later).
Insulin has been in clinical practice for 100 years, originally isolated from canine pancreas in Canada and later from bovine and porcine sources. Exogenous insulin interacts with the insulin receptor similarly to endogenous insulin to produce therapeutic effects. The first Australian patient received insulin in 1923 at the age of 6. She ultimately lived a long life and passed away at the age of 81.
Things have changed quite a lot over the last 100 years and the advent of recombinant DNA technology in the 1980s led to the development of synthetic human insulin and insulin analogues.
Going back to the ‘basal’ and ‘bolus’ concept, much effort has been made to alter the peptide chains of exogenous insulin to produce pharmacokinetics that better replicate the pancreatic insulin release characteristics in health individuals. Substitutions of amino acids within the A and B chain tails have been utilised to adjust the onset and duration of action of various insulin analogues.
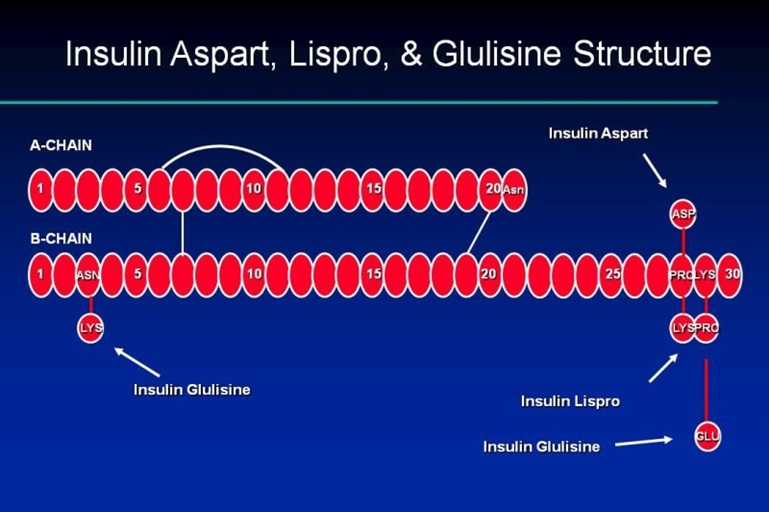
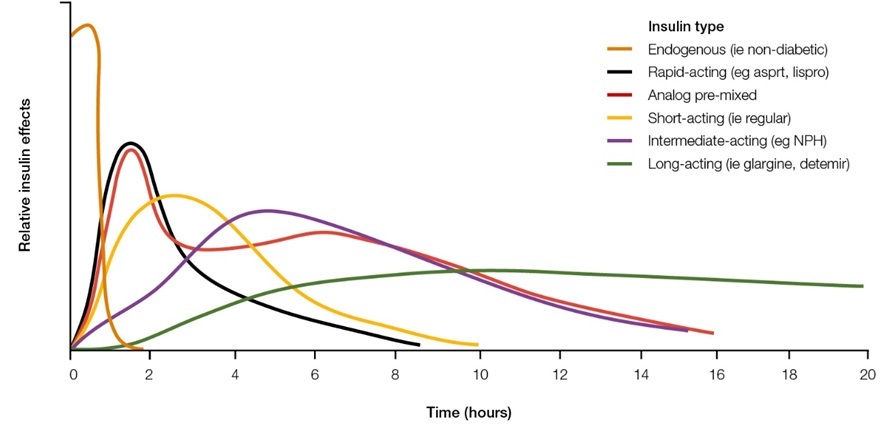
These modifications have resulted in a variety of different insulin formulations. They are typically classified according to their duration of action (rapid, intermediate, or long-acting). Some insulin products contain a mixture of both rapid and intermediate or long-acting insulin (pre-mixed).
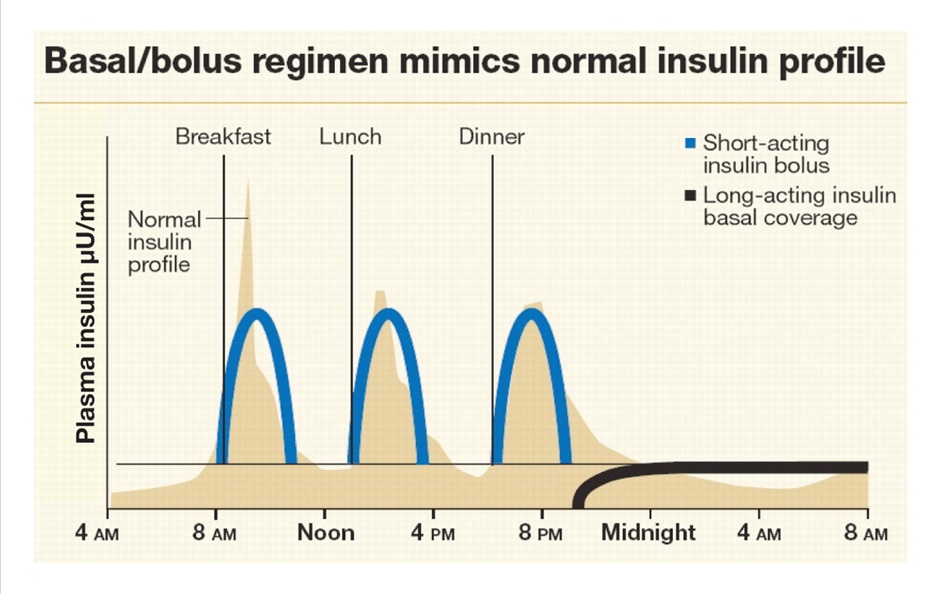
Considering insulin release in the healthy individual, you can see how a combination of a long-acting insulin such as insulin glargine once daily and rapid acting insulin such as insulin aspart prior to meals begins to emulate normal insulin release. This results in 4 injections per day. In addition to insulin injections, patients will be doing a finger prick BGL test prior to insulin administration (in most circumstances). Premixed insulins can reduce the number of injections per day as they are often administered only twice daily but don’t replicate normal insulin release as well.
Explore the different types of insulins available in Australia using the link below. You will not be expected to remember all the different types of insulin available, but we will cover some common examples in the GLS that are important to remember.
‘Traditional’ insulin devices
Traditionally, insulin is administered as a subcutaneous injection into the abdomen. This is typically via an ‘insulin pen’ but there are other devices available for those with vision impairment or needle phobia. Insulin can also be administered into other sites of adipose tissue including the thighs, buttocks and arms.
Watch the following video showing the use of a Flexpen device.
To maximise shelf life, insulin is stored under refrigeration 2-8oC. Insulin can be stored at <30oC for up to 28 days, this is called the ‘in-use’ stability. Injecting cold insulin typically stings more than room temp. Patients must be instructed to not freeze insulin and if frozen, the insulin should be discarded. Freezing may alter the peptide structure resulting in unpredictable effects or lack of efficacy.
Each injection of insulin requires a new needle (something a lot of patients don’t do). Leaving the needle on an insulin pen and reusing it increases the likelihood of scarring and lipohypertrophy which can alter the absorption of insulin from subcutaneous tissue and it is also more painful to use a blunt needle. Good injection technique and rotation of the injection site are very important to help avoid lipohypertrophy and inadvertent intramuscular injection.
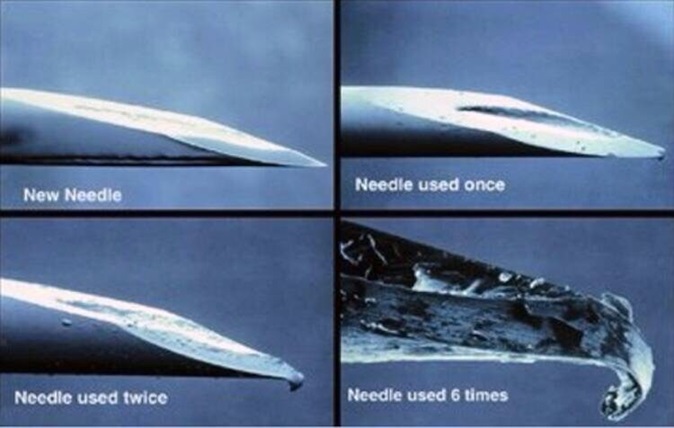
Fortunately, insulin needles and other consumables are subsidised via the National Diabetes Services Scheme.
Insulin Pumps and Continuous/Flash Glucose Monitors
The burden of multiple times per day injections and BGL monitoring can be significant. Insulin pumps and continuous blood glucose monitors can help to overcome this and their uptake amongst T1DM patients is increasing.
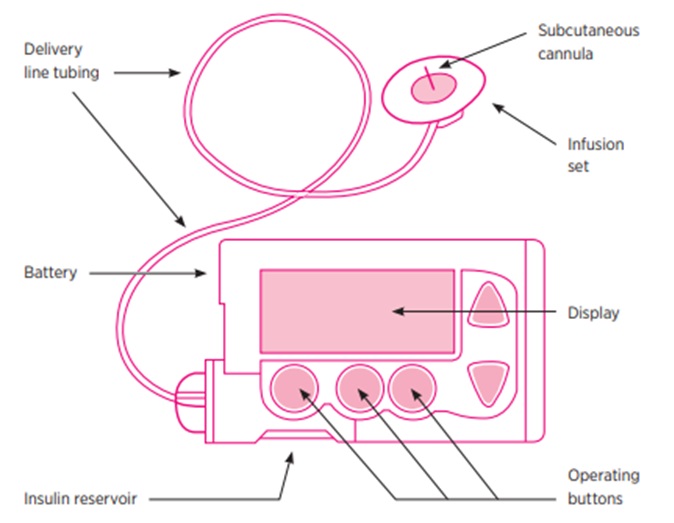
The insulin pump delivers a continuous background infusion of a rapid acting insulin and then deliver a bolus dose when eating. Both are delivered via the same subcutaneous cannula. The cannula and tubing are typically replaced every 3 days.
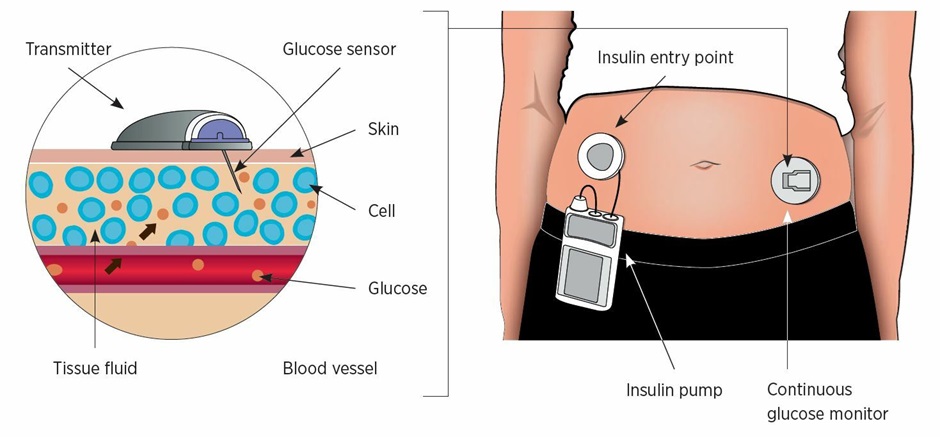
Insulin pumps, continuous/flash glucose monitors and smartphones continue to improve. Over the last few years significant advances have been made making them easier to manage and ‘smart’ functions have become available that assist with insulin dosing calculations.
Despite the apparent advantages, these devices are not suitable for all patients and they require an individualised assessment to determine their utility in a specific patient.
Non-BGL Insulin Indications
Before we move onto non-insulin therapies used in T2DM we will quickly look at some uses of insulin that are not related to BGL control.
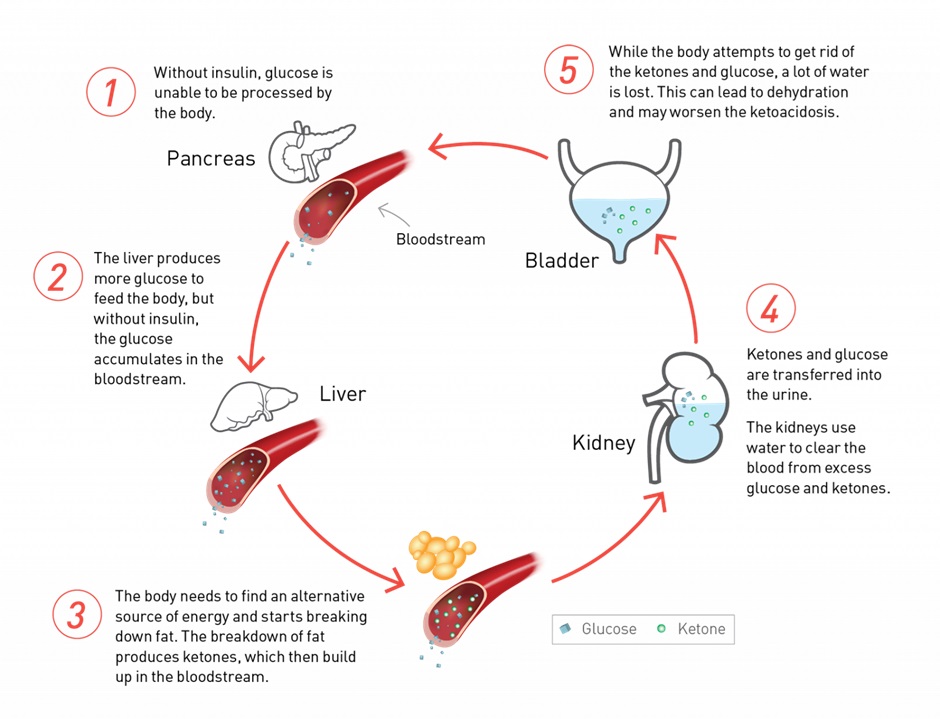
As we have discussed earlier, insulin not only reduces BGLs but it also reduces ketone body formation. This is important to remember as it forms the basis of diabetic ketoacidosis management alongside fluid and electrolyte replacement.
Insulin is infused intravenously to prevent further ketone formation and correct hyperglycaemia. The hyperglycaemia is generally corrected quite quickly but ketone bodies persist. A glucose infusion is commenced alongside the insulin infusion to prevent life threatening hypoglycaemia while the insulin infusion is continued until ketone bodies have dropped sufficiently via renal and other forms of clearance.
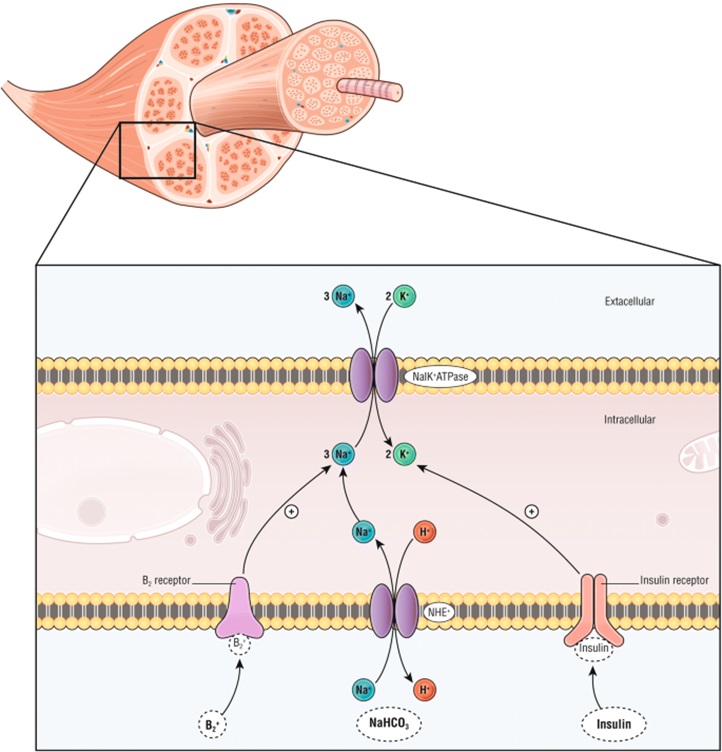
Another common use of insulin in acute care is the management of hyperkalaemia. Insulin binding to insulin receptors leads to activation of the Na+/K+ ATPase in skeletal muscle resulting in an intracellular shift of potassium and subsequent reduction in serum levels. It is important to give glucose alongside the insulin to prevent hypoglycaemia. Beta-2 agonists (from week 1) also trigger the Na+/K+ ATPase and salbutamol is often used alongside insulin to reduce serum potassium levels. In severe hypokalaemia we also give intravenous calcium to help stabilise the myocardium and prevent arrythmia formation.
Check Your Understanding:
- Exogenous insulin and endogenous insulin work in the same way.
- Insulin increases the uptake of glucose from the blood by cells via increased expression of GLUT-4.
- Insulin decreases the release of glucose from the liver and increases the storage of glucose for later use.
- Insulin is capable of producing significant BGL reductions but it is also the most prone to cause hypoglycaemia.
- Insulin analogues and devices have been developed to minimise the risk of hypoglycaemia, better mimic physiological insulin release and reduce the burden for patients.
Watch this video – Insulin Therapy
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING
This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.
