6.3 Oral Hypoglycaemic Use in Diabetes
The Ominous Octet
It is important to understand some of the physiological changes that occur in T2DM. This helps to identify treatment targets and aids in the understanding of pharmacology.
Historically, the understanding of T2DM focussed on insulin resistance in muscle and liver cells and impaired insulin release from the pancreas. The pathophysiology of T2DM is complex and in 2008 the ominous octet was proposed to help better describe the mechanisms behind hyperglycaemia in T2DM.
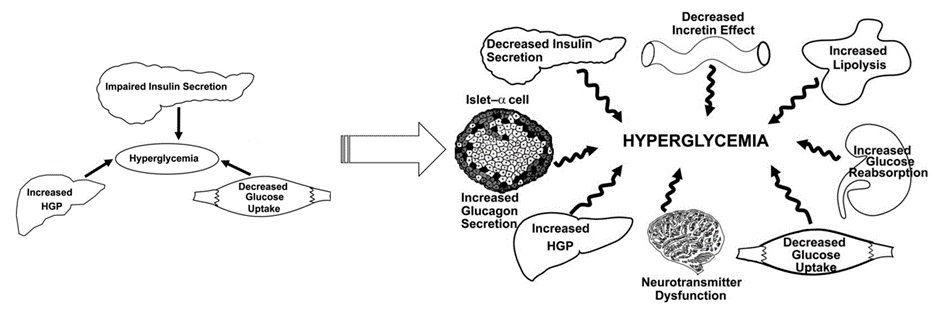
The ominous octet introduced the concepts of reduced incretin effect, increased lipolysis, increased glucose reabsorption, neurotransmitter dysfunction and alpha-cell functions as contributors to hyperglycaemia in T2DM. Some of these effects had been known for many years but had not yet yielded pharmacotherapies.
As you move through the T2DM content, you will notice that the ominous octet forms the backbone of currently available pharmacotherapy including the newer therapies including SGLT-2 inhibitors, DPP-4 inhibitors and GLP-1 receptor agonists.
As research into T2DM continues, we will undoubtedly see an increase in the number of potential targets for disease control beyond the ominous octet.
Watch this video – Ominous Octet
Metformin
Metformin is the only biguanide hypoglycaemic agent available (currently). It is typically the first choice in T2DM as it reduces blood glucose levels with a low inherent risk of hypoglycaemia. Metformin also reduces appetite (with a slight weight reduction benefit). The UKPDS study in the late 1990s provided evidence of cardiovascular benefits in overweight T2DM patients.
Mechanism of action of metformin (a biguanide)
Metformin has two main effects resulting in the reduction of blood glucose levels:
- It suppresses hepatic glucose production (probably the main effect).
- It reduces insulin resistance by increasing the uptake of glucose into the liver and peripheral tissues.
It may also have a role in reducing the uptake of glucose from the small intestine and reducing lipolysis.
Metformin and hepatic gluconeogenesis (action 1)
The cellular mechanism for the reduction of hepatic glucose occurs through down regulation of transcription factors that facilitate gluconeogenesis (the production of glucose from other sources).
Metformin increases AMPK (AMP-activated protein kinase), this in turn causes the decreased expression of the genes responsible for coding of the enzymes that cause glucose production via gluconeogenesis (and thus hepatic glucose production).
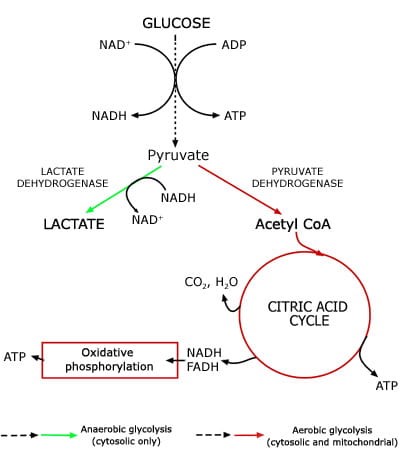
The increase in AMPK also causes an increase in the conversion of pyruvate to lactate. The reduced availability of pyruvate reduces hepatic glucose production (glucose is synthesized from the breakdown of pyruvate amongst other things) via gluconeogenesis. The important thing to remember here is, in the liver, metformin reduces the substrate for gluconeogenesis and the expression of genes of gluconeogenesis.
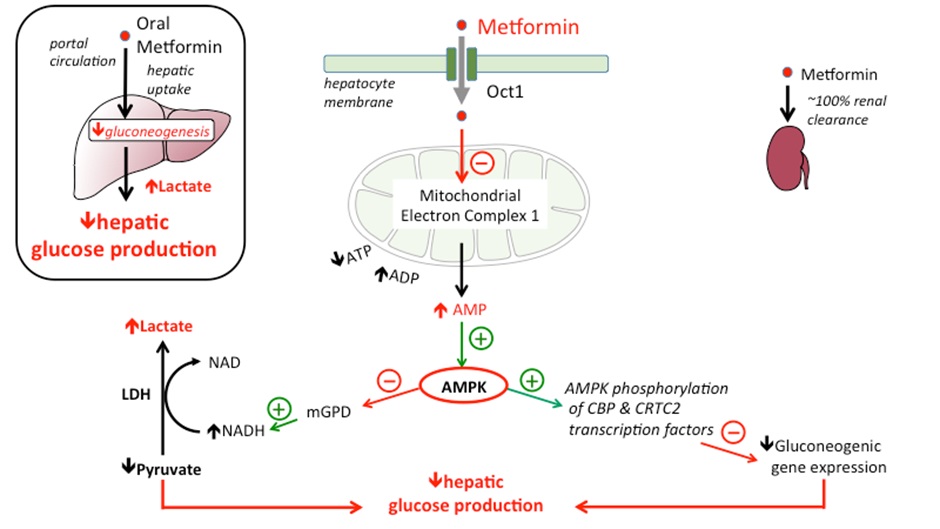
The other effect of this process is to increase the availability of lactate (pyruvate is broken down into lactate and Acetyl CoA for entry into the Krebs cycle or converted into glucose by gluconeogenesis via another mechanism). It is the increased availability of lactate that theoretically exposes patients to increased risk of lactic acidosis (discussed later).
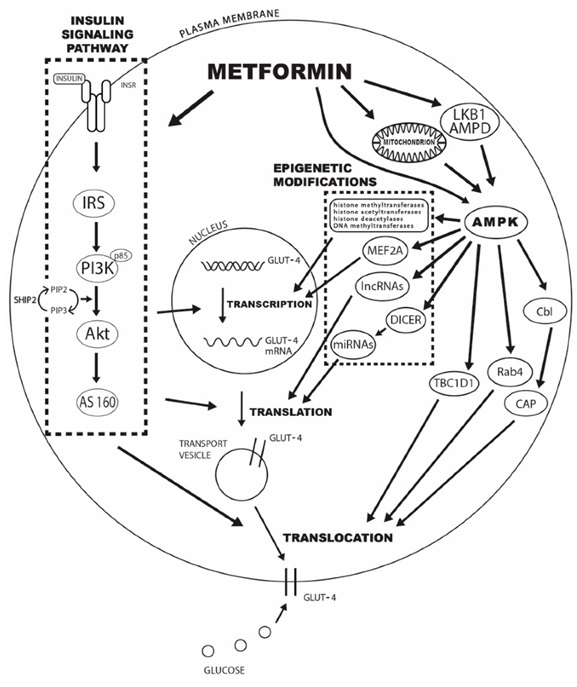
Metformin and Glucose Transport (action 2)
The second major blood-glucose lowering effect is the increase in the expression of GLUT-4 protein in peripheral tissues (remember this from insulin). The increase in AMPK directly increases the expression of GLUT-4 protein. This increases the movement of glucose from the blood into the cell and therefore decreases circulating levels of glucose.
In T2DM, there is an insulin resistance that can limit insulin effects. As you can see in the image below, the action of metformin increases GLUT-4 translocation independent of the insulin-insulin receptor pathway helping to increase peripheral glucose uptake.
Side effects and precautions of metformin
The main side effects of metformin include nausea, anorexia, cramps and diarrhea. It also causes a decrease in the absorption of Vitamin B12. The gastrointestinal side effects can be minimised by taking with food and starting at a low dose and increasing slowly as tolerated.
Lactic acidosis is a rare event and the link between metformin and lactic acidosis is not clear. It occurs in about 4 in 100,000 patient years (that’s not much in anyone’s language). A Cochrane review failed to find evidence of an increased risk of lactic acidosis in patients treated with metformin compared to other T2DM therapies.
Given the mechanistic action of metformin, if a patient was to be susceptible to lactic acidosis, the patient characteristics would be:
- Receiving a very high metformin doses
- Significant renal impairment
- Increases accumulation of metformin in the body
- Reduces lactic acid clearance
- Risk factors for acute kidney injury
- Other risk factors for acidosis including
- Hepatic failure
- Respiratory failure
- Myocardial infarction or decompensated heart failure
- Alcohol excess
- Sepsis
- Elderly
If you are interested, there is an interesting podcast that explores many aspects of metformin including lactic acidosis available here.
Check your understanding:
- Metformin reduces hepatic glucose production and increases peripheral glucose uptake – both actions reduce circulating blood glucose levels.
- Increased lactate is a theoretical consideration – risks of lactic acidosis increase with advanced age, hepatic dysfunction, renal dysfunction, or condition that simulate renal failure and high doses of metformin.
- Dose is reduced with declining renal function.
- The incidence of hypoglycaemia with metformin is very low.
- The most common side effects of metformin are gastrointestinal in nature and include nausea, anorexia, cramps and diarrhea.
- Vitamin B12 deficiency can occur with metformin.
Watch this video – Metformin
Sulfonylureas
Sulfonylureas are also known as secretagogues. They act by increasing the release of insulin from beta-cells in the pancreas. For sulfonylureas to be effective, there must be functioning beta-cells. As time goes by and beta-cells are lost as a natural consequence of the progression of diabetes, the sulfonylureas become less effective.
The 4 sulfonylureas are:
Gliclazide (Gli-cla-zide)** **most frequently used.
Glipizide (Glip-e-zide)
Glibenclamide (Gli-ben-clam-ide)
Glimepiride (Gli-mep-e-ride)
Sulfonylurea mechanism of action
In normal healthy beta-cells, glucose transport into the beta-cell causes the release of insulin via the ATP-mediated inhibition of the ATP-sensitive potassium channel. Sulfonylureas also block the ATP-sensitive potassium channel (KATP). This increases the accumulation of potassium within the cell causing a depolarization of the cell. This depolarization triggers the voltage sensitive calcium channel. The resultant influx of calcium into the cell causes the binding of insulin vesicles to the cell wall and release of insulin.
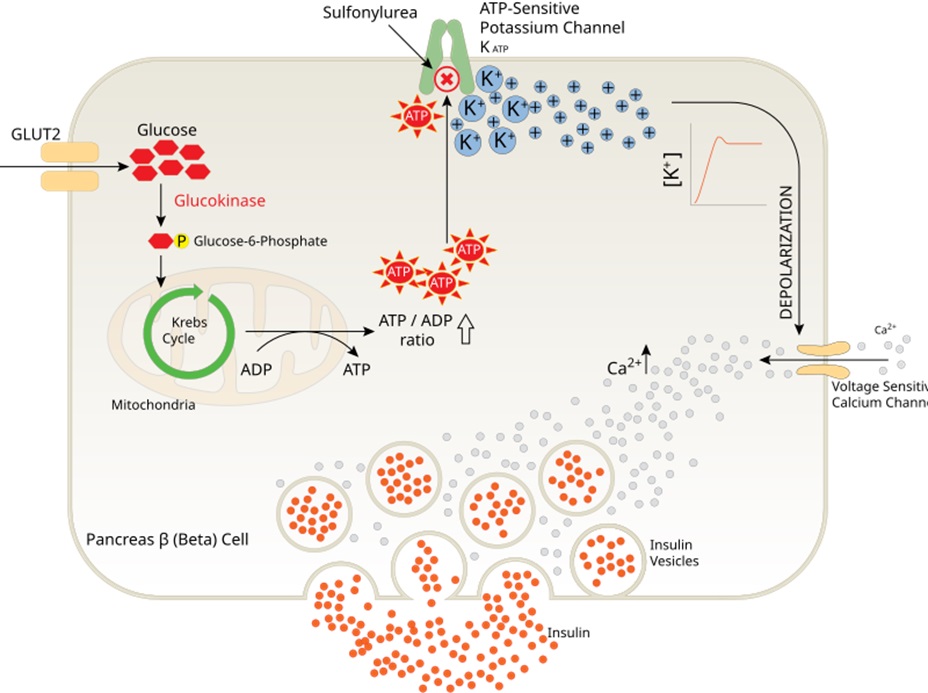
Side effects associated with sulfonylureas
Historically sulfonylureas were considered an alternate first line drug in the management of T2DM alongside metformin. Their use is now declining because of the common unwanted effects and risks associated with this class of drug. These include:
- Weight gain. Patients are at risk of an average of 2-2.3kg weight gain.
- Risk for severe hypoglycaemia. Sulfonylureas have the highest risk of severe hypoglycaemia compared to all other oral hypoglycaemic agents. The risk of hypoglycaemia increases with patient age, renal and hepatic dysfunction.
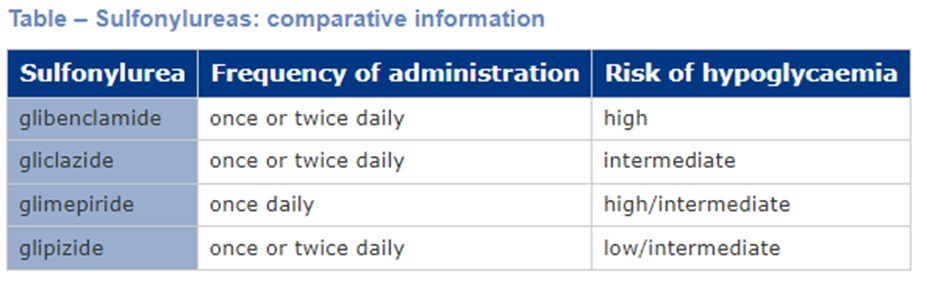
- Cardiovascular risk. Sulfonylureas lack cardiovascular benefits. There is some conflicting evidence regarding a possible increase in cardiovascular risk when using sulfonylureas. On balance, the increase in cardiovascular risk is likely related to not using an agent with proven benefit rather than inherent increased risk with sulfonylureas.
- Sulfonylurea efficacy declines with time. Sulfonylurea efficacy is entirely dependent upon functioning beta-cells. This means as beta-cell function declines as inevitably does with diabetes progression, the effectiveness of sulfonylureas decreases.
Due to the risks of hypoglycaemia, lack of cardiovascular benefits and weight gain, obese patients are not normally prescribed sulfonylureas. Their use is also reducing in other cohorts of patients for these reasons.
Practice points related to hypoglycaemia
- Gliplizide has the lowest risk of hypoglycaemia.
- Glibenclamide has the highest risk of hypoglycaemia
- Taking with food decreases risk of hypoglycaemia. Alcohol increases the risk of hypoglycaemia.
- Sulfonylureas are metabolised by CYP2C9. Concomitant administrator with CYP2C9 inhibitors increases the risk of hypoglycaemia.
Check your understanding:
- Sulfonylureas increase insulin secretion from pancreatic beta-cells independent of BGL.
- Sulfonylureas possess the highest risk of hypoglycaemia amongst the oral T2DM therapies. The risk differs between patients and between drugs in this class.
- Sulfonylureas lack cardiovascular benefits and lead to weight gain and are now used less frequently with the release of newer T2DM therapies.
Watch this video – Sunfonylureas
Sodium-glucose co-transporter-2 inhibitors (SGLT-2i)
The sodium-glucose co-transporter-2 inhibitors (AKA glucuretics or SGLT2i) are a relatively new class of hypoglycaemic medication. They increase urinary excretion of glucose leading to a reduction in BGL. SGLT-2 inhibitors are now one of the first medication classes added to metformin for management of T2DM.
There are currently two drugs available in Australia:
Dapagliflozin (dapa-glif-lozin
Empagliflozin (empa-glif-lozin)
You should remember SGLT-2 inhibitors from heart failure in week 3.
Sodium-glucose co-transporter-2 inhibitor mechanism of action
Under normal conditions, glucose is filtered by the glomerular apparatus and the filtered glucose is quickly reabsorbed in the proximal convoluted tubule by two pumps – the Sodium-glucose co-transporter-2 (SGLT-2) and the Sodium-glucose co-transporter-1.
The sodium-glucose co-transporter-2 pump is located in the proximal section of the proximal tubule. SGLT2 facilitates glucose and sodium movement across the apical membrane of the tubular epithelial cell. GLUT2 then transports glucose across the basolateral membrane and into the peritubular capillary.
90% of filtered glucose is reabsorbed via SGLT2. The remaining 10% of glucose is reabsorbed by SGLT1 in the distal section of the proximal convoluted tubule. This means that under normal circumstances all filtered glucose is reabsorbed.
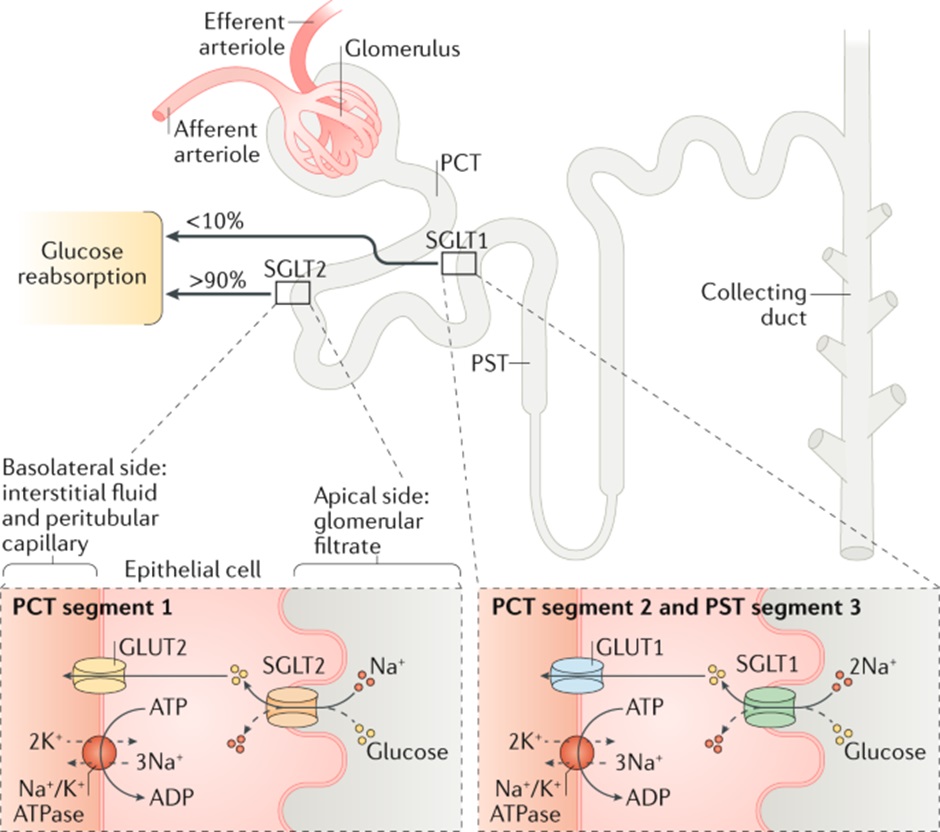
Mechanism of action of SGLT-2 Inhibitors
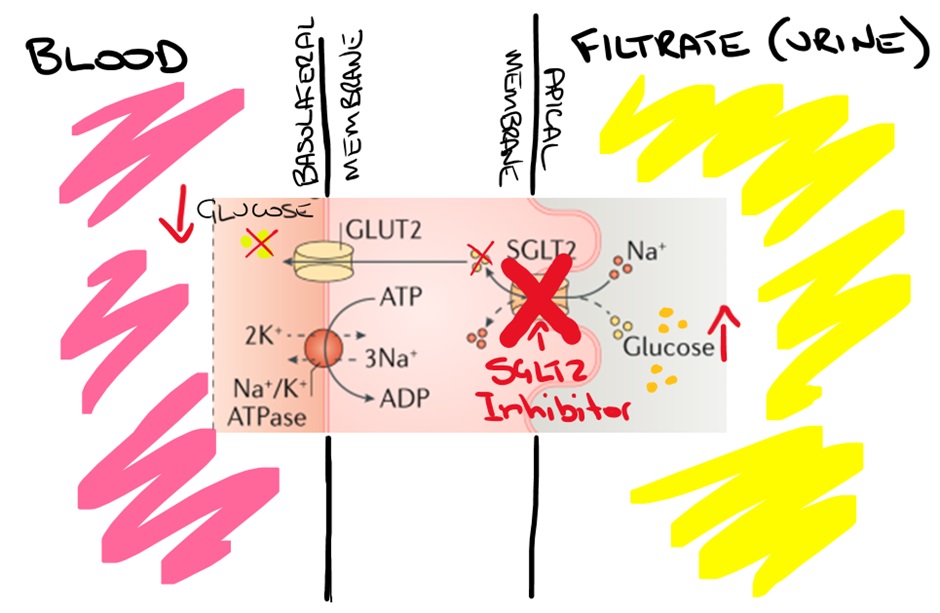
SGLT-2 inhibitors block the SGLT-2 pump in the proximal section of the proximal convoluted tubule. This significantly decreases the reabsorption of glucose by the proximal section of the proximal convoluted tubule, increasing glucose excretion in the filtrate.
The increased excretion of glucose in the urine (glucosuria) reduces blood glucose concentrations. The effect also increases the amount of water excreted. Because the osmotic concentration of the urine is increased, driven by the elevated glucose concentration of the filtrate, osmotic diuresis occurs. The figure below shows the effect of blockade of the SGLT-2 pump increasing the excretion of glucose in the filtrate.
The BGL lowering effects of SGLT-2 inhibitors require adequate renal function. As renal function declines, the effect on BGL reduces. Despite this, the other numerous advantages persist, and SGLT-2 inhibitors are now commonly used down to relatively low eGFRs of approximately 20-30ml/min (or lower).
Benefits of SGLT-2 inhibitors
There are several benefits of SGLT-2 inhibitors. These drugs have a relatively low risk of hypoglycaemia, they provide a modest reduction in weight secondary to increased lipolysis and decreased body fat, they lower blood pressure and decrease cardiovascular risk and are reno-protective. Remember this is what we are trying to achieve when treating diabetes!
In addition to the endocrine benefit of reducing plasma glucose levels and lower risk of hypoglycaemia, CV benefits can be explained by the reduction in circulating blood volume, reducing systolic blood pressure, reducing preload and afterload and the direct downregulation of sympathetic activity amongst other poorly understood benefits. The figure below relates the reduction in circulating volume (secondary to the osmotic diuresis that occurs via the glucosuria) and the reduction of elevated sympathetic activity.
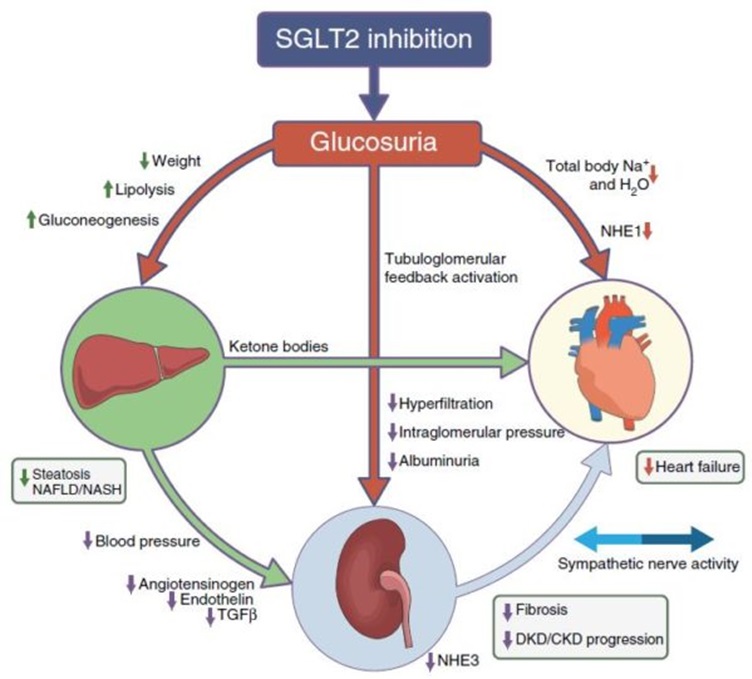
General side effects of SGLT-2 inhibitors
There are several side effects related to SGLT-2 inhibitors. The most common side effects relate to glucosuria and include:
- Increase in genital infections (vulvovaginal candidiasis, balanitis) secondary to glucosuria which creates a good environment for bacterial and fungal growth
- Dehydration
- Hypotension and volume depletion
- Constipation, thirst
- Polyuria, dysuria and urinary tract infections (UTI less common than thrush)
- A reduction in eGFR when starting
- Haemoconcentration and reduction in glomeruli pressure which is reversible
- Hypoglycaemia
- When used in combination with insulin or sulfonylurea (no surprises)
There are also some signals that SGLT-2 inhibitors lead to increase fracture risk. Appropriate monitoring of bone mineral density is prudent.
The scary one – Euglycaemic diabetic Ketoacidosis (edka)
Typically, diabetic ketoacidosis (yes, we’re still talking about this) presents with an elevated BGL however it can also occur during euglycaemia (‘normal’ BGL). Probably the most alarming side effect of SGLT-2 inhibitors is the increased risk of euglycaemia ketoacidosis.
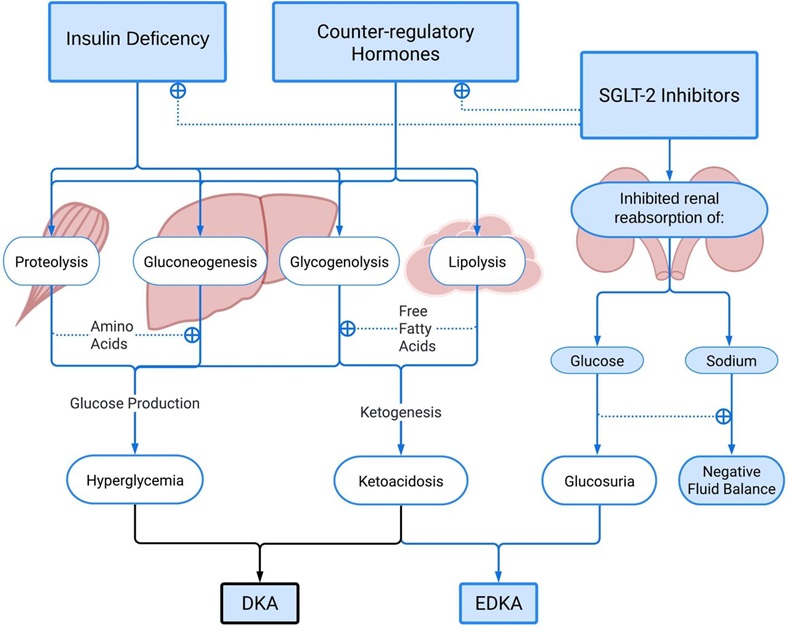
Fortunately, EDKA occurs very rarely (0.1-0.5% of patients receiving SGLT-2 inhibitors). There are some important factors that increase the risk including:
- Intercurrent illness
- Can reduce oral intake
- You can also get an increase in pro-ketogenic counter-regulatory hormones released during illness (same as DKA)
- Low carb intake
- Fasting (eg prior to surgery)
- Colonoscopy preparation
- Excessive alcohol consumption
To minimise the risk of ketoacidosis, patients should be instructed not to take their SGLT-2 inhibitor prior to surgery, when they feel unwell (esp vomiting, diarrhoea, fever) and if they are not eating and drinking normally. They can generally restart once they are eating and drinking normally again.
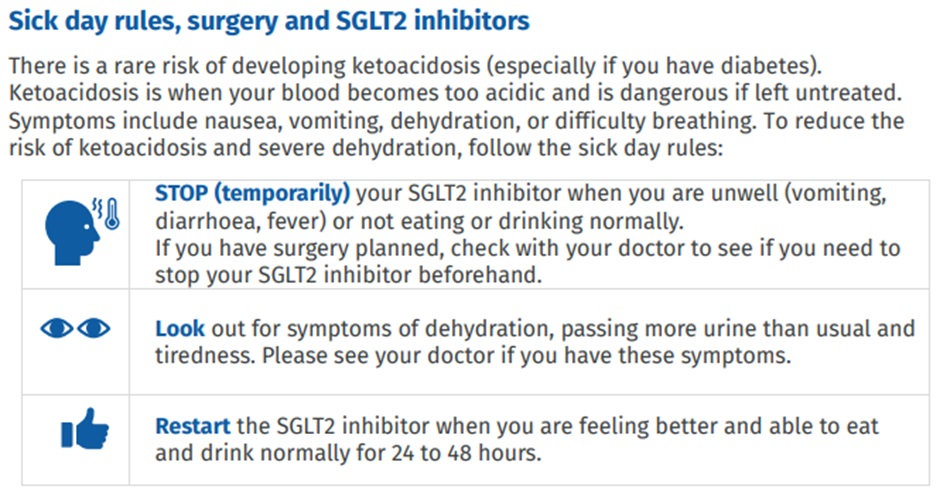
Check your understanding:
- SGLT-2 inhibitors reduce the reabsorption of glucose from the filtrate in the proximal tubule increasing urinary excretion of glucose and lowering blood glucose levels.
- SGLT-2 inhibitors have cardiovascular and reno-protective benefits separate to BGL reductions.
- Many of the side effects are due to the osmotic diuresis caused by increased glucosuria (genital infections and dehydration).
- Euglycaemic ketoacidosis is a potential risk that requires appropriate counselling including sick day management.
- Becoming the standard add-on therapy to metformin (like GLP-1 RAs).
Watch this video – Inhibitors
Role of incretins in the regulation of blood glucose levels
The incretins are small intestine-derived hormones that stimulate the reduction of blood glucose. Incretins are so called as they increase the release of insulin (amongst other things). Incretins are released in L and K cells in the gut in response to fat and glucose ingestion.
Patients with T2DM have a reduced incretin effect ultimately leading to increased blood glucose levels. The two main types of incretin hormones are:
- GLP-1Glucagon-like peptide-1
- GIPGlucose-dependent insulinotropic peptide AKA gastric inhibitory peptide
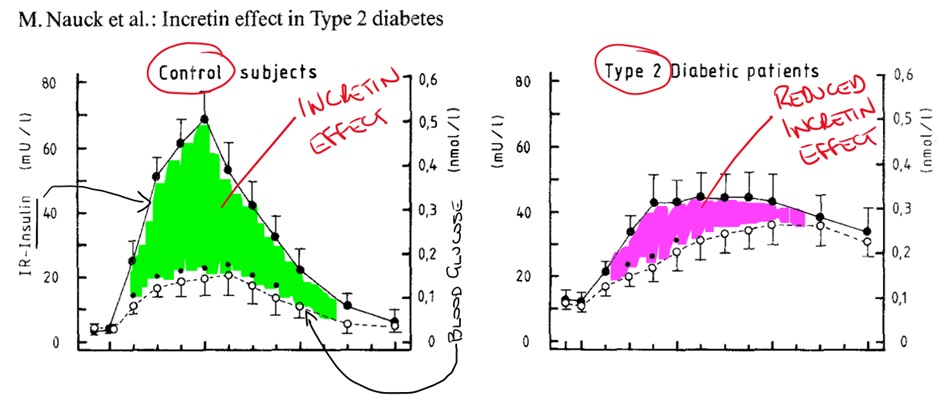
GLP-1 and GIP are released in response to fat and glucose ingestion. GLP-1 increases insulin release from the beta-cells in the pancreas, decreases glucagon release from the alpha-cells of the pancreas and decreases appetite (increases satiety). GLP-1 also increases beta-cell proliferation and decrease beta-cell apoptosis.
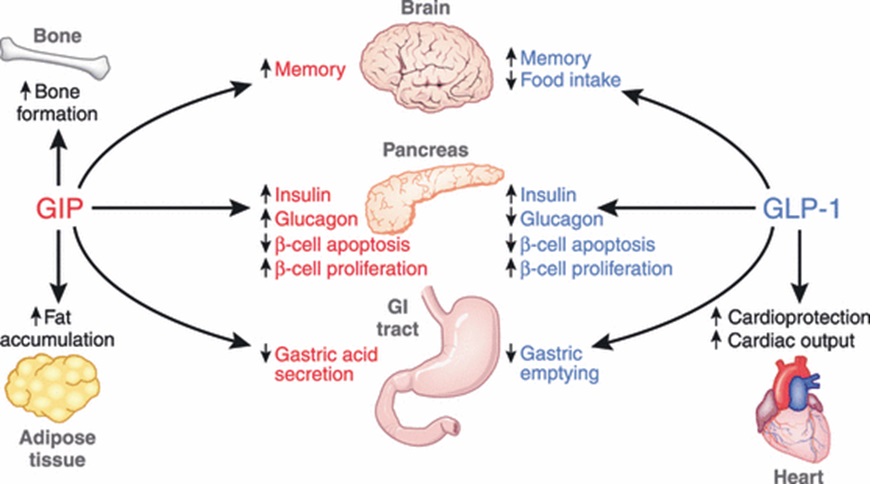
GIP has a similar effect on insulin secretion, beta-cell apoptosis and proliferation. It also increases glucagon production. Overall, incretin hormones increase insulin secretion, increase beta-cell proliferation, decrease beta-cell apoptosis and have variable effect on glucagon.
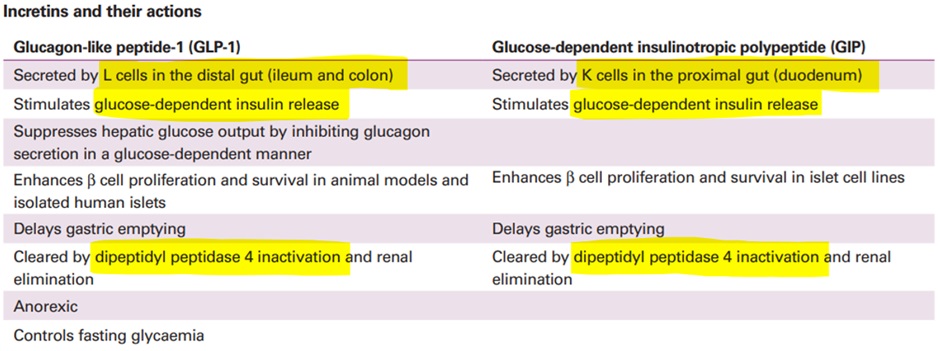
The half-life of the incretins is relatively short – in the range of 1-3 minutes. Both GLP-1 and GIP are inactivated by the enzyme dipeptidyl peptidase-4 (DPP-4).
Watch this video – Incretins
DPP-4 Inhibitors (gliptins)
There are 5 oral dipeptidyl peptidase-4 inhibitors:
- Sitagliptin * * most commonly seen in practice
- Linagliptin *
- Alogliptin
- Vildagliptin
- Saxagliptin
They all share the suffix ‘gliptin’ and for this reason are known as the gliptins. The gliptins reduce the breakdown of the incretins (GLP-1 and GIP) increasing glucose dependent insulin release and decrease glucagon release. The figure below shows the effect of DPP-4 inhibitors on the amount of active GLP-1 and GIP. The net effect is to increase insulin release and inhibit glucagon release – overall reducing blood glucose levels.
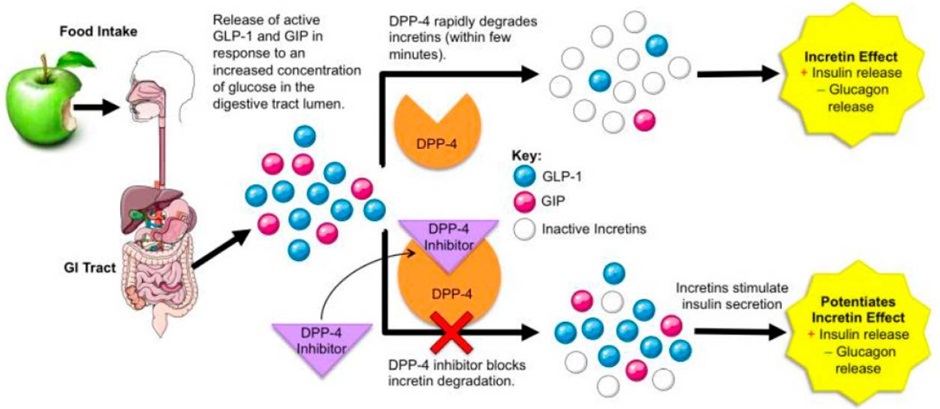
Despite the multiple potential benefits of GLP-1 and GIP, the primary effect of DPP-4 inhibitors in clinical practice appears to be a reduction in BGL. The reduced incretin effect seen in T2DM (reduced GIP sensitivity and reduced GLP-1 release) does not appear to be significantly overcome by DPP-4 inhibitors.
DPP-4 inhibitors have demonstrated cardiovascular safety (some questions remain for saxagliptin) but have failed to show cardiovascular benefits. They also have no significant impact on weight.
Side effects of DPP-4 inhibitors
The DPP-4 inhibitors are generally well tolerated with minimal side effects. Hypoglycaemia is uncommon except when used in combination with insulin or sulfonylureas. Headache and musculoskeletal pain are possible which can be quite troubling. Pancreatitis and other pancreatic complications have been linked to DPP-4 inhibitor use however the available evidence suggests this risk is very low.
Check your understanding:
- Inhibit the action of DPP-4 thus increasing the action of the incretins GLP-1 and GIP.
- Increase the release of insulin from the beta-cells and decreases the release of glucagon from the alpha-cells in the pancreas.
- No little/no risk of hypoglycaemia (when not given with exogenous insulin or sulfonylureas).
- Are generally well tolerated but some caution in patients with prior pancreatitis.
- Have not been shown to improve cardiovascular outcomes and have no significant impact on weight.
Watch this video – Dipeptidyl Peptidase-4 Inhibitors ‘gliptins’
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.
