7.1 Thyroid and Bone
Thyroxine, T3 and Antithyroid agents, and Osteoporosis
Learning Outcomes
Be able to:
- Describe the role of the thyroid gland and thyroid hormones in maintaining normal cellular and organ function
- Know the pharmacological difference between T3 or T4.
- Identify the type of receptor T3 or T4. act upon.
- Describe the signs and symptoms of too much
- Describe the action of drugs used to reduce excess thyroid function.
- Describe the drugs used in osteoporosis.
Thyroid gland disorders
The thyroid gland is a very important endocrine gland critical for normal development and maintenance of many systems. Disorders of the thyroid gland typically manifest as excessive thyroid horomone release or insufficient thyroid hormone release. This module will briefly explore the pharmacology of the drugs used to treat thyroid disease. To understand the function of these drugs, it is important to understand the structure and function of the thyroid gland, how thyroid hormone is manufactured by the body and the role of thyroid hormone in normal development and homeostasis.
Revision of the thyroid gland
The thyroid gland is located in anterior of the neck, over the trachea, below the larynx. It is a bi-lobed organ, joined by a bridge of tissue (isthmus) and is “butterfly shaped”. It has a purely endocrine function, is highly vascular and soft in consistency. It has four parathyroid glands (producing parathyroid hormone – calcium, magnesium and phosphate regulation).
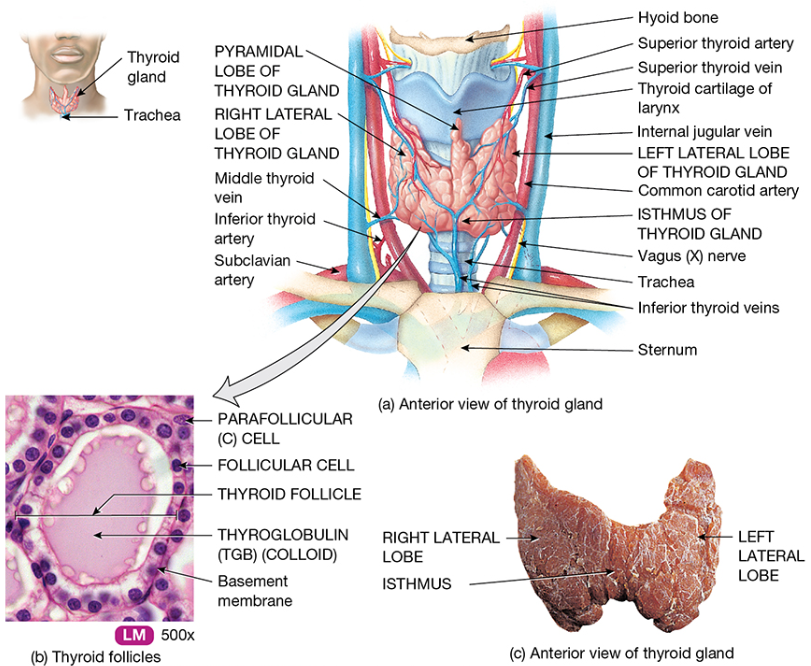
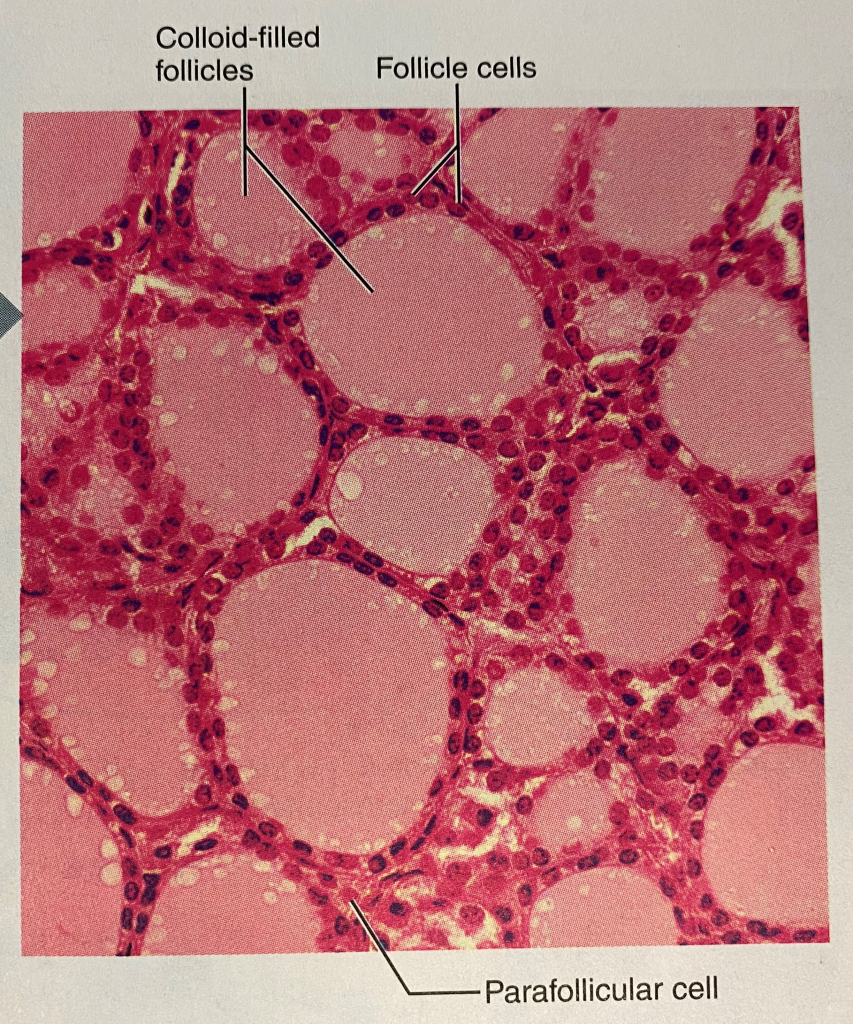
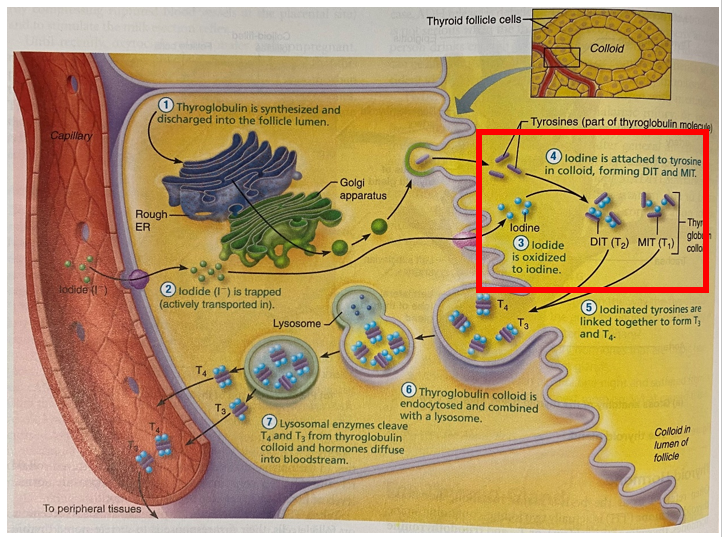
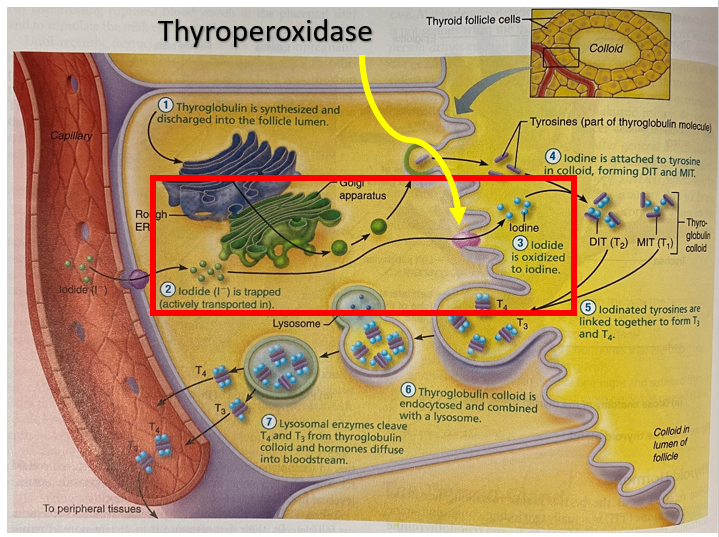
At the microscopic level the thyroid gland is composed of hollow spherical follicles that produce the glycoprotein thyroglobulin. Thyroglobulin and iodine atoms are stored in the follicle – this will later be modified to thyroid hormone.
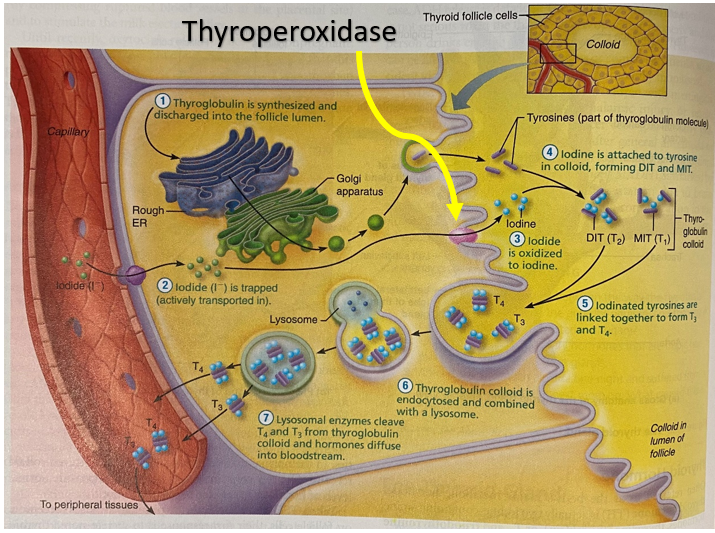
Production of thyroid hormone by the thyroid gland
Iodide (I–) is actively transported into the thyroid follicle. It is converted to Iodine (I) by the enzyme thyroperoxidase and attached to tyrosine to form either
- T1 = the combination of one iodine and a tyrosine (monoiodotyrosine (MIT))
- T2 = the combination of two iodines and a tyrosine (diiodotyrosine (DIT))
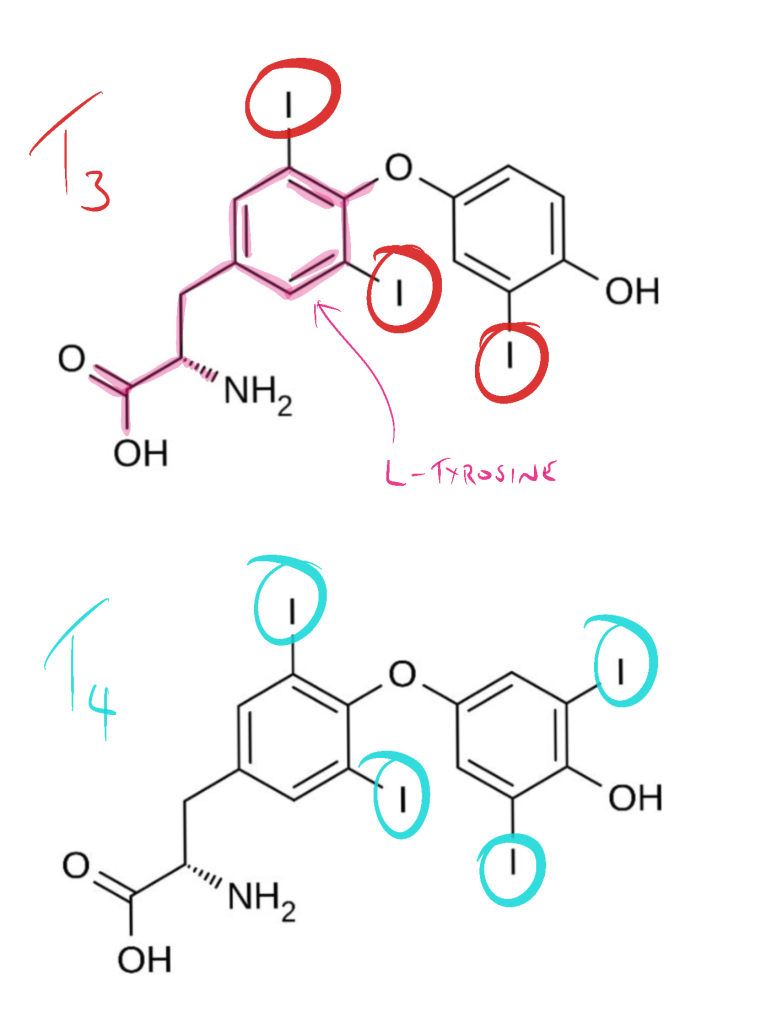
The iodinated tyrosine molecules are linked together to produce two types of iodine-containing thyroid molecules (hormones) – T3 and T4:
- MIT + DIT = T3
- DIT + DIT = T4
Triiodothyronine (T3) is the “active” thyroid hormone and is mainly generated in the periphery following deiodination of T4. The chemical difference between T3 and T4 is simply the number of iodine molecules though the biological differences between the two could not be more different with T4 providing no relevant biological activity and T3 responsible for all of the biological activity of the two thyroid hormones. The chemical differences between the two molecules are shown below.
Thyroid hormone function
Thyroid hormone binds to intracellular nuclear receptors to induce gene transcription. As thyroid hormone is ‘permissive’, in the absence of thyroid hormone binding to the promotor/regulatory regions of the target genes, those genes are under- or not expressed.
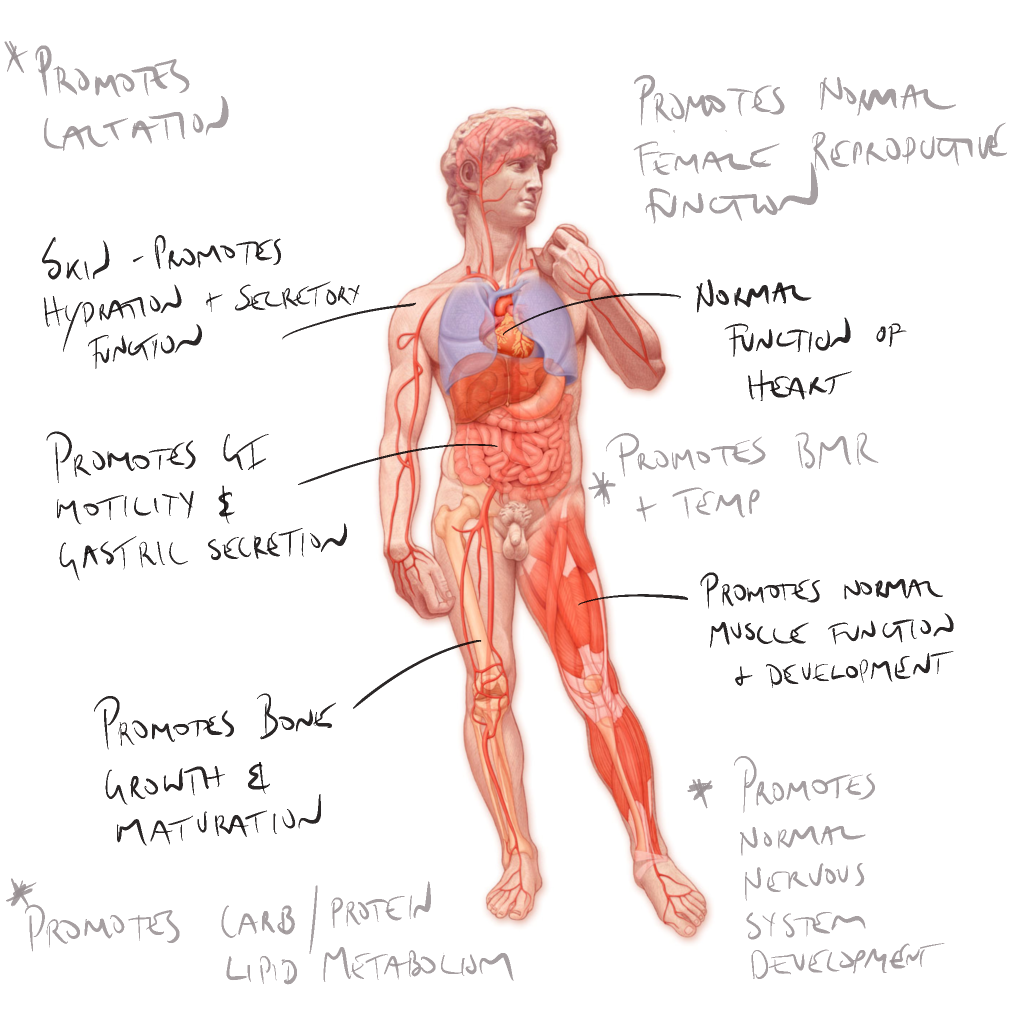
Thyroid hormone (TH) acts in the developed body to:
Metabolic:
- Maintain thermogenic and metabolic homeostasis: makes physiological processes thermodynamically inefficient, promoting heat production;
- Set basal metabolic rate (BMR) / body temperature – promotes normal oxygen use and BMR, calorigenesis, enhances the effect of sympathetic nervous system;
- Nutrient metabolism and glucose catabolism, mobilises fats, necessary for protein synthesis; and
- Enhances liver production of cholesterol.
Organ system:
- Promotes normal growth and maturation of skeleton;
- Facilitates normal cardiovascular, skin and gastrointestinal function; and
- Promote increased body calcium storage.
Developmental:
- Cellular and neurological development:
- Important role in cellular differentiation and CNS development during foetal development; and
- Critical role in immediate post-partum development of the brain, and general development of the musculoskeletal and reproductive systems.
📺 Watch the vodcast on thyroid gland structure and function (9 minutes).
Summary
- Thyroid hormones are produced by the thyroid gland
- Two types of thyroid hormone are produced, the biologically active T3 and the pro-hormone (biologically inactive) T4
- The thyroid gland produces more inactive T4 than active T3. T4 (inactive) is converted to the active T3 at the target organ
- Thyroid hormone is responsible for regulation of many organs – a very important hormone. They regulate reproductive capability, temperature, CNS development, energy metabolism, GIT function, sensitivity to SNS
Thyroid gland disorders
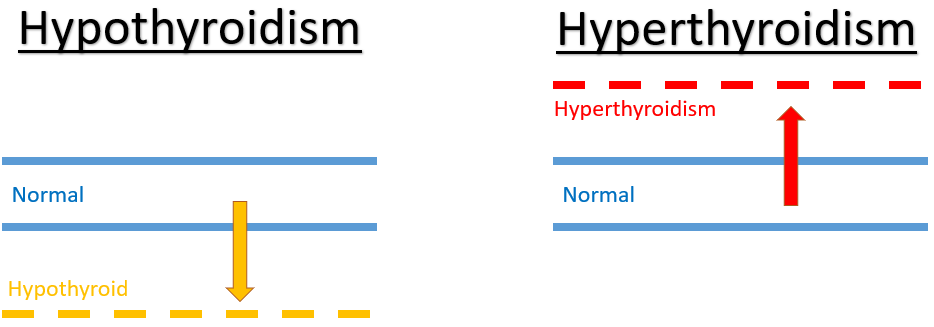
There are two main types of thyroid disorders:
- Underproduction of thyroid hormone by the thyroid gland results in low thyroid hormone levels. This is known as HYPOTHYROIDISM and is treated with exogenous replacement thyroid hormone thyroxine (T4).
- Excessive production of thyroid hormone levels is known as HYPERTHYROIDISM. This condition is treated with thyroid gland suppression medicines carbimazole and propylthiouracil.
To manage low thyroid hormone production, the strategy is simple – replace the missing thyroid hormone with a synthetic version. There are two types of thyroid hormone:
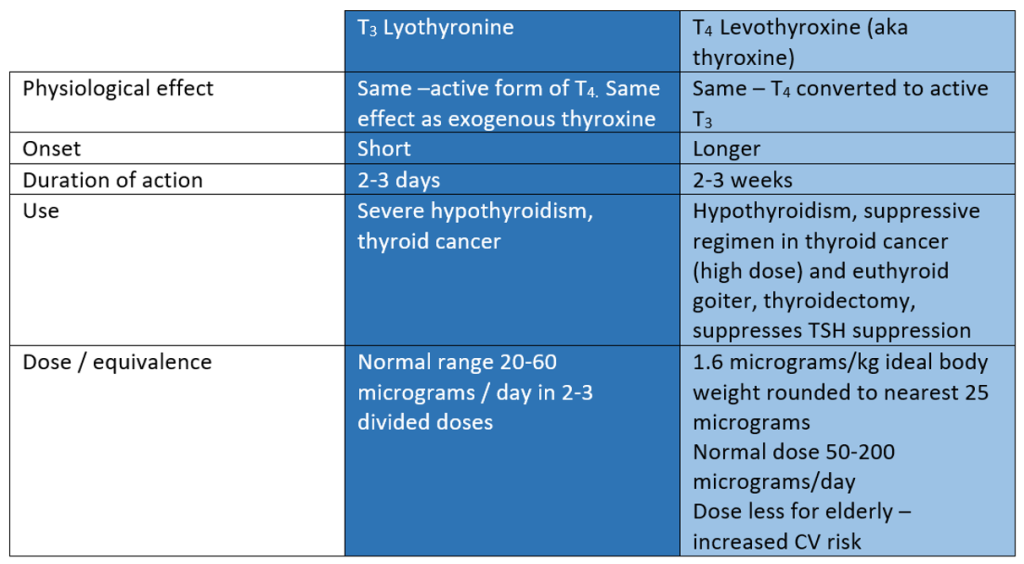
- Levothyroxine (T4) – preferred for replacement therapy
- Liothyroxine (tri-iodothronine, T3) – shorter half-life – more potent – used for emergency use
While the therapeutic effect of exogenous T3 and T4 converted to T3 are the same, there are some important clinical differences between the two drugs that explain why thyroxine (T4) is the preferred thyroid replacement strategy in hypothyroid disease. The table below compares T3 (Lyothyronine) and T4 (Levothroxine – aka thyroxine).
Where excessive thyroid hormone production is encountered, thyroid gland production of thyroid hormone can be reduced and peripheral conversion to T4 (inactive) to T3 (active) can also be reduced. There are two drugs used to suppress the production and activity of thyroid hormones:
- Carbimazole and
- Propylthiouracil (PTU)
Carbimazole
Carbimazole is a pro-drug, converted to methimazole. Methimazole competitively inhibits thyroid peroxidase enzyme from coupling iodine to thyroglobulin and forming MIT or DIT. This reduces the production of T3 and T4. The figure below shows (in the red box) the step that joins thyroglobulin to iodine. Inhibition of the formation of MIT and DIT reduces the production of T3 and T4.
Propylthiouracil (PTU)
Propylthiouracil (PTU) inhibits thyroperoxidase, the enzyme that oxidises iodide to iodine. The reduction of available iodine reduces the production of T3 and T4 in the thyroid gland. Propylthiouracil also inhibits the conversion of T4 to T3 in the peripheral tissues.
📺 Watch the vodcast on thyroid disorders and treatment (15 minutes)
Osteoporosis (OP)
Risk Factors for Osteoporosis
The single non-modifiable risk factor for OP is a history of parental fracture. The non-modifiable and modifiable risk factors for OP include:
| Non-modifiable risk factors for OP | Modifiable risk factors for OP |
|---|---|
| Premature menopause | Low physical activity |
| Hypogonadism | Low body weight |
| Multiple falls | Protein or calcium under-nutrition |
| Poor balance | Smoking |
| Alcohol >2 standard drinks per day | |
| Vitamin D insufficiency |

A number of medications have had a negative effect on bone density. Medications with a large and modest negative effect on BMD include:
| Medicines with a LARGE negative effect on BMD | Medicines with a MODEST negative effect on BMD |
|---|---|
| Glucocorticoids of duration greater than 3 months and dose of equal to or more than 7.5mg/day | SSRIs |
| Excess thyroid hormone replacement | Anti-psychotics |
| Aromatase inhibitors | Thiazolidenediones |
| Anti-androgen therapy. | Anti-epileptic medications |
| PPIs |
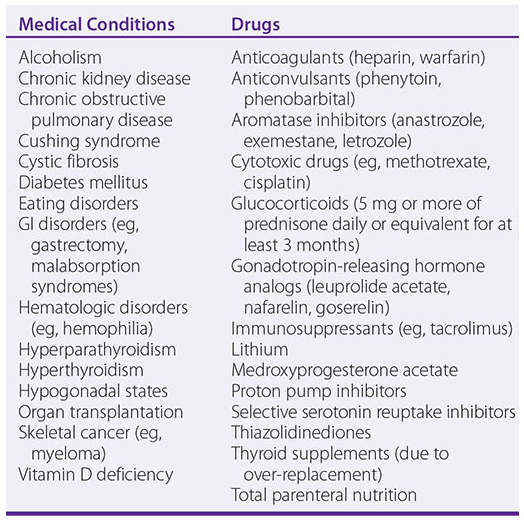
Medical conditions and Drugs Associated with Osteoporosis
Management of Osteoporosis
Despite the success of anti-resorptive therapy, and the relative safety and strong cost-benefit of therapy available, only a minority of patients with OP are treated with pharmaceutical therapies. That is not to say the only therapy for osteoporosis and minimal-trauma fracture is drug therapy alone. For primary and secondary prevention of OP and minimal-trauma fracture, risk factors should be addressed, management of comorbidities should be optimised and the use of drugs that reduce bone homeostasis or density should be minimised where possible. Key strategies to reduce the risk include:
- implement strategies to prevent falls
- increase weight-bearing exercise and balance training
- ensure adequate calcium intake
- ensure vitamin D sufficiency
- stop smoking
- limit alcohol intake to two standard drinks per day
- maintain ideal body weight.
Drugs Used in Osteoporosis
Two types of agents are currently used for treatment of osteoporosis:
- Antiresorptive drugs that decrease bone loss, e.g. bisphosphonates, calcitonin, selective [o]estrogen receptor modulators (SERMs), denosumab,
- Anabolic agents that increase bone formation, e.g. PTH, teriparatide.
We will discuss only two important drugs used in osteoporosis, bisphosphonate and denosumab.
Bisphosphonates
Bisphosphonates are analogues of pyrophosphate, a normal constituent of tissue fluids that accumulates in bone and has a role in regulating bone resorption. The examples include alendronate, risedronate, and zoledronic acid. Bisphosphonates primarily target osteoclasts to inhibit bone resorption. These drugs are used long term for prevention and treatment of osteoporosis, and for symptomatic Paget’s disease.
Bisphosphonates – Mechanism of Action
These compounds bind strongly to calcium within the bone matrix, forming stable complexes. These complexes are then taken up into osteoclasts during bone resorption and inhibit several enzymes in the mevalonate biosynthetic pathway. This process decreases prenylation of proteins required for osteoclast function and survival and inhibits osteoclast bone resorption. As osteoclasts resorb bone, the bisphosphonates are gradually released, leading to high localized concentrations that impede the activity of these bone-resorbing cells.
Bisphosphonates – Pharmacokinetics
Oral bisphosphonates should be taken on an empty stomach with a full glass of water, while sitting or standing, at least 30 minutes before breakfast due to the risk of severe esophageal irritation. Pamidronate and zoledronate, on the other hand, are given intravenously. These drugs have low gastrointestinal absorption, with around 50% of the absorbed dose accumulating at bone mineralization sites. There, they attach to hydroxyapatite crystals and can remain for months or even years until the bone undergoes resorption. The unbound portion of the drug is excreted unchanged through the kidneys.
Bisphosphonates – Adverse Effects
Common adverse effects include nausea, vomiting, diarrhoea, headache, hypocalcaemia, musculoskeletal pain. Some patients may infrequently experience oesophagitis, oesophageal erosions and ulcers (mainly with alendronate), gastritis, duodenitis, glossitis, rash
Denosumab
Denosumab is a human monoclonal antibody (mab) that targets and inhibits receptor activator of nuclear factor kappa B ligand (RANKL), the primary signal for bone resorption. RANKL is an important regulator of osteoclast development and activity. Denosumab prevents RANKL binding to its receptor (RANK) on osteoclasts surface reducing osteoclast formation, function and survival, which results in decreased bone resorption and increased mass and strength.
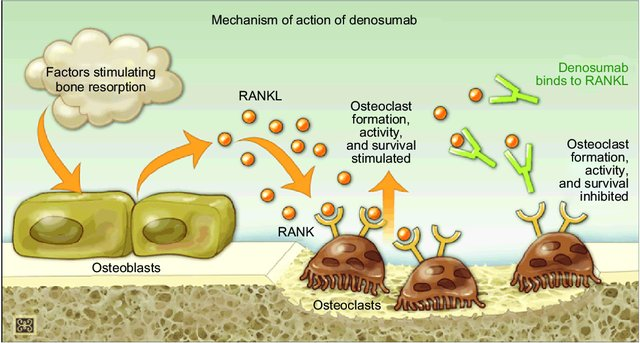
Denosumab is particularly beneficial when bisphosphonates are unsuitable. This medication is approved for use in men and postmenopausal women with osteoporosis who are at high risk of fractures. Additionally, Denosumab is used to prevent skeletal-related complications in patients with bone metastases from solid tumors and to manage bone loss in individuals undergoing hormone ablation therapy for breast or prostate cancer. Before initiating treatment with Denosumab, it’s essential to correct calcium and vitamin D deficiencies and complete any necessary dental procedures to mitigate the risk of osteonecrosis of the jaw, a potential side effect also associated with potent bisphosphonates. Denosumab is administered via subcutaneous injection, with a dosage of 60 mg every six months for women with postmenopausal osteoporosis or men at increased risk of osteoporosis due to hormone ablation, and more frequently (monthly) for patients with bone metastases.
Common side effects include gastrointestinal disturbances (diarrhea or constipation), shortness of breath, hypocalaemia in renal impairment, low phosphate levels, infections (such as respiratory or ear infections, cellulitis), or skin rash, with rare instances of osteonecrosis of the jaw.
📺 Watch the vodcast on Osteoporosis and its treatment (7 minutes)
Summary
- Excessive thyroid hormone production is termed hyperthyroidism
- Low thyroid hormone production is termed hypothyroidism
- Hypothyroidism is treated by supplementing the missing thyroid hormone
- there are two forms of thyroid hormone – The biologically active T3 and the pro-hormone (biologically inactive T4
- T4 is the preferred thyroid hormone replacement strategy – it is converted to T3 (active form) in the peripheral tissues
- Where excessive thyroid hormone is produced, thyroid hormone production is reduced by either blocking the coupling of thyroglobulin and iodine to form MIT and DIT as is the case of carbimazole OR
- The process of I– oxidation is interrupted by inhibiting thyroperoxidase (as is the case for PTU) and blocking the peripheral conversion of T4 to T3.
📓 Lecture Notes
MD2012_W7_Lecture 1_Thyroid_and_Bone
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING
This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.