7.2 Endocrine: Reproductive Hormones
Reproductive Hormones, Contraceptives and Menopausal Hormone Therapy (MHT)
Learning Outcomes
By the end of this module, it is expected students should be able to demonstrate and apply knowledge of:
- Release mechanism of reproductive hormones i.e., testosterone, oestrogen and progesterone from gonads
- Main roles/functions of reproductive hormones
- Endogenous testosterone, therapeutic uses of synthetic analogues and their toxicities
- Application of synthetic oestrogen and progesterone in contraception and hormone replacement therapy (HRT).
The Hormones – a brief overview
The hypothalamic-pituitary-gonadal (HPG) axis includes the hypothalamus, anterior pituitary gland and gonads. Gonadotrophins are the hormones produced to control the reproductive system.
Hormonal control of the reproductive systems in men and women involves sex steroids from the gonads (ovaries and testis), hypothalamic mediators including the decapeptide gonadotrophin releasing hormone (GnRH), and glycoprotein gonadotrophins from the anterior pituitary gland. Kisspeptin, a protein that is a G protein–coupled receptor ligand for a receptor known as GPR54, initiates secretion of GnRH at puberty. GnRH is released from the hypothalamus to act on the anterior pituitary triggering the release of luteinising hormone (LH), and follicle-stimulating hormone (FSH). These gonadotrophic hormones control sexual maturation and gametogenesis.
Gonadotropin Releasing Hormone (GnRH) – GnRH initially stimulates synthesis of FSH and LH; continuous administration of GnRH agonists inhibits gonadotrophin production, suppressing ovarian and testicular steroidogenesis.
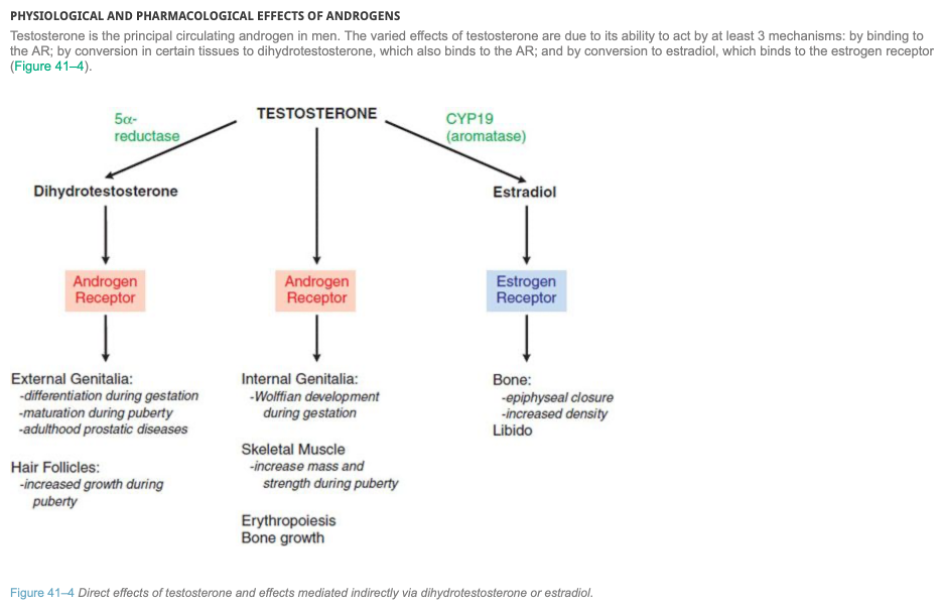
Testosterone is the main male sex steroid. It is synthesised mainly by the interstitial cells of the testis, and in smaller amounts by the ovaries and adrenal cortex. Testosterone binds to and activates androgen receptors that regulate androgen-responsive genes involved in the development and maintenance of masculine sexual characteristics. It also affects libido and has systemic anabolic effects. Testosterone can be converted in skin, gonadal tissue and the prostate gland in men to dihydrotestosterone by 5α -reductase. In the bone and brain, testosterone is converted by aromatisation into oestradiol. High levels of testosterone have a negative feedback effect on the hypothalamus to inhibit GnRH production, as well as on gonadotrophs in the pituitary to inhibit LH secretion, thereby decreasing testosterone levels.
Estrogen – Estrogens are synthesised by the ovary and placenta, and in small amounts by the testis and adrenal cortex. The starting substance for synthesis of estrogen and other steroids is cholesterol. There are three main endogenous estrogens in humans: oestradiol, oestrone and oestriol. Oestradiol is the most potent and is the principal estrogen secreted by the ovary.
Effects of exogenous estrogen in females depend on the state of sexual maturity when the estrogen is administered: In primary hypogonadism: estrogen stimulates the development of secondary sexual characteristics and accelerates growth. In adults with primary amenorrhoea: estrogen, given cyclically with a progestogen, induces an artificial cycle. In sexually mature women: estrogen (with a progestogen) is contraceptive. At or after menopause: estrogen replacement prevents menopausal symptoms and bone loss.
Estrogen binds to nuclear receptors, as do other steroid hormones. There are at least two types of estrogen receptor, termed ER α and ER β. Natural and synthetic estrogens are well absorbed in the gastrointestinal (GI) tract, but after absorption the natural estrogens are rapidly metabolised in the liver, whereas synthetic estrogens are degraded less rapidly. There is variable enterohepatic cycling. Most estrogens are readily absorbed from skin and mucous membranes. They may be given as intravaginal creams or pessaries for local effect.
Progestogens – The natural progestational hormone (progestogen) is progesterone. This is secreted by the corpus luteum in the second part of the menstrual cycle, and by the placenta during pregnancy. Small amounts are also secreted by the testis and adrenal cortex. The density of progesterone receptors is controlled by estrogens. Progesterone itself is virtually inactive orally, because of presystemic hepatic metabolism. Other derivatives are available for oral administration, intramuscular injection or administration via the vagina or rectum.
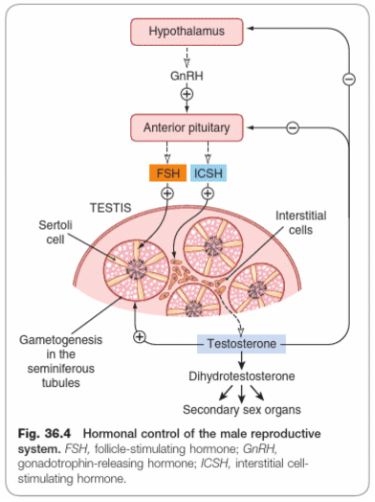
GnRH controls the secretion of gonadotrophins by the anterior pituitary. This secretion is not cyclical as in menstruating women, although it is pulsatile in both sexes.
The secretion of luteinizing hormone (referred to as interstitial cell-stimulating hormone (ICSH) in the male) and FSH from the anterior pituitary by GnRH regulates spermatogenesis. LH/ICSH secretion begins at puberty, and the consequent secretion of testosterone causes maturation of the reproductive organs and development of secondary sexual characteristics. ICSH stimulates the synthesis of testosterone by binding to LH receptors on the Leydig cells of the testis, triggering a cascade of enzymatic reactions converting cholesterol to testosterone. Testosterone diffuses into the bloodstream to be distribute to the peripheral tissues, where FSH stimulates Sertoli cells to stimulate spermatogenesis.
FSH drives sperm production in the Sertoli cells of the testes, as well as synthesis of proteins important for the production and action of steroid hormones. They include:
- Androgen binding protein (ABP) which maintains high levels of testosterone locally in the luminal space of the seminiferous tubules.
- P450 aromatase, an enzyme that converts testosterone into oestradiol.
- Growth factors that support sperm cells and spermatogenesis, that result in increasing the number of sperm cells, as well as promote motility and the fertility potential of sperm.
- Inhibins which have a selective negative feedback effect on FSH only and not LH (i.e., inhibits FSH production, but does not inhibit LH production). They also act as growth factors on Leydig cells.
Testosterone has a feedback effect on the anterior pituitary, modulating its sensitivity to GnRH and thus influencing secretion of LH/ICSH. Testosterone has marked anabolic effects. Secretion of testosterone is mainly controlled by LH/ ICSH, but FSH also plays a part, possibly by releasing a factor similar to GnRH from the Sertoli cells which are its primary target.
👁️🎧Watch this YouTube video about testosterone production:
TESTOSTERONE
In male hypogonadism, androgen replacement with testosterone is important to prevent long-term complications, such as fragility fractures, and to maintain sexual function and overall wellbeing. Androgens (also known as anabolic steroids and including ones not marketed in Australia (eg nandrolone, oxandrolone, oxymetholone) are misused by some athletes and body builders in an attempt to increase muscle mass; this exposes these people to an increased risk of serious adverse effects.
DRUGS FOR BENIGN PROSTATIC HYPERPLASIA –
Inhibit 5‑alpha-reductase, which converts testosterone to dihydrotestosterone (a potent cellular androgen that stimulates prostate growth). They reduce prostate size and improve symptoms and urinary flow rate.
ANTI-ANDROGENS
E.g Cyproterone – Competitively inhibit the binding of androgen at androgen receptors. Cyproterone (a steroidal anti-androgen) also has progestogenic effects that result in decreased production of testosterone. Nonsteroidal anti-androgens do not suppress androgen production and may even increase testosterone concentration.
📺 Watch the brief vodcast on the introduction to the Reproductive Hormones, testosterone and anti-androgen (15 minutes)
Hormonal control of the Female Reproductive System
Hormonal control of the female reproductive system is slightly more complicated and occurs cyclically.
Some key points:
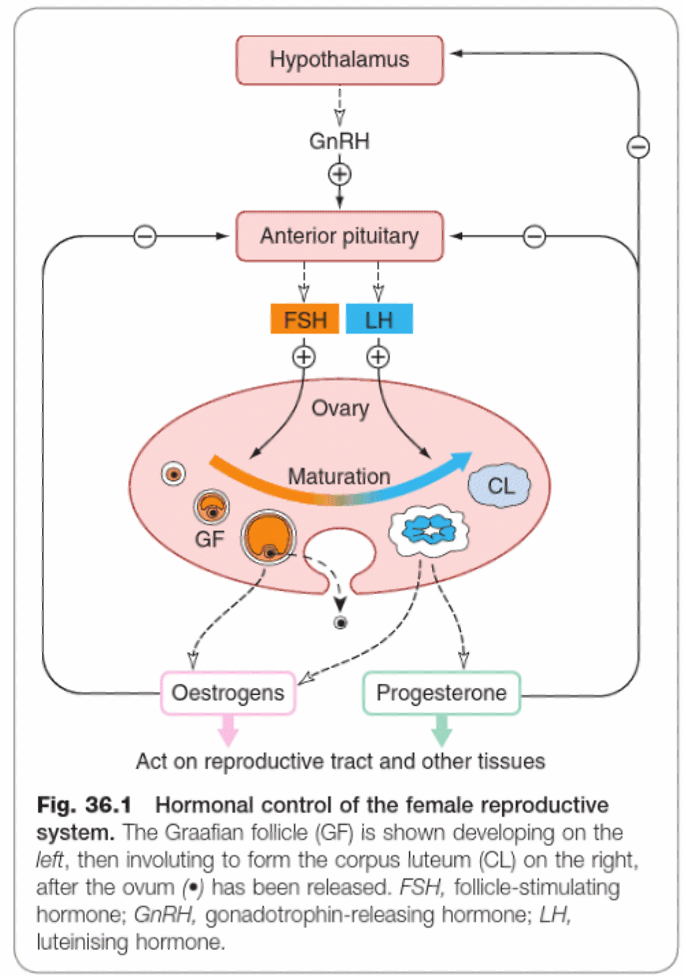
- GnRH released from the hypothalamus, acts on the anterior pituitary to release FSH and LH. FSH and LH stimulate follicle development in the ovary.
- FSH is the main hormone stimulating estrogen release.
- LH stimulates ovulation at mid-cycle and is the main hormone controlling subsequent progesterone secretion from the corpus luteum.
- Estrogen controls the proliferative phase of the endometrium and has negative feedback effects on the anterior pituitary.
- Progesterone controls the later secretory phase and has negative feedback effects on both the hypothalamus and anterior pituitary.
- If a fertilised ovum is implanted, the corpus luteum continues to secrete progesterone. After implantation, human chorionic gonadotrophin (HCG) from the chorion becomes important, and later in pregnancy progesterone, HCG and other hormones are secreted by the placenta.
👁️🎧 Watch this YouTube video about estrogen and progesterone production:
LH and FSH stimulate the ovary to produce mature gametes, as well as synthesise and secrete oestrogens and progestins.
LH binds to theca cells on developing follicles as well as granulosa cells. After ovulation, LH binds to cells of the corpus luteum. It acts on theca cells to produce progestins and androgens. Androgens enter granulosa cells and are then converted to oestrogens.
FSH binds to granulosa cells to:
- Increase production of enzymes that catalyse the production of steroid hormones, stimulating follicle growth
- Increase production of activins, which have a positive feedback effect on the anterior pituitary
- Increase production of inhibins, which have a selective negative feedback effect on the pituitary
- Help convert androgens to oestrogen.
Feedback Effects in Females
Oestrogens and progestins act on the anterior pituitary and the hypothalamus to exert negative and positive feedback effects.
- Moderate oestrogen levels exert negative feedback on LH and FSH secretion.
- High oestrogen levels (in the absence of progesterone) positively feedback on LH and FSH secretion.
- Oestrogen in the presence of progesterone exerts negative feedback on the HPG axis.
Progesterone increases the inhibitory effect of moderate oestrogen concentration levels on LH and FSH secretion. However, progesterone prevents the positive feedback effect of high oestrogen concentrations on the pituitary.
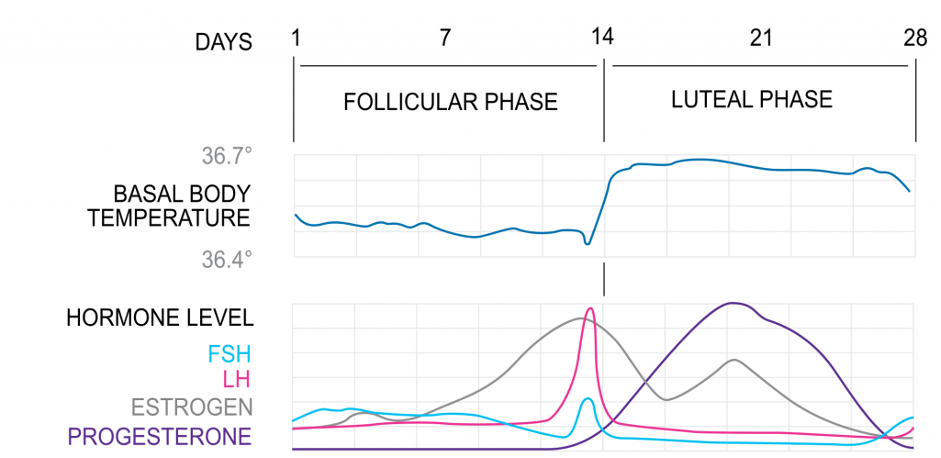
1. Follicular Phase
The follicular phase marks the beginning of a new cycle as follicles (oocytes surrounded by stromal cells) begin to mature and prepare to release an oocyte. At the start of a new cycle (menses) there is little ovarian hormone production and the follicle begins to develop independently of gonadotropins or ovarian steroids. Due to the low steroid and inhibin levels, there is little negative feedback at the HPG axis, resulting in an increase in FSH and LH levels. These stimulate follicle growth and estrogen production. Only one dominant follicle can continue to maturity and complete each menstrual cycle. As estrogen levels rise, negative feedback reduces FSH levels, and only one follicle can survive, with the other follicles forming polar bodies. Follicular estrogen eventually becomes high enough to initiate positive feedback at the HPG axis, increasing levels of GnRH and gonadotropins. However, the effect is only reflected in LH levels (the LH surge) due to the increased follicular inhibin, selectively inhibiting FSH production at the anterior pituitary. Granulosa cells become luteinised and express receptors for LH.
The uterine proliferative phase runs alongside the follicular phase, preparing the reproductive tract for fertilisation and implantation. Estrogen initiates fallopian tube formation, thickening of the endometrium, increased growth and motility of the myometrium and production of a thin alkaline cervical mucus (to facilitate sperm transport).
In response to the LH surge, the follicle ruptures and the mature oocyte is assisted to the fallopian tube by fimbria. Here it remains viable for fertilisation for around 24 hours. Following ovulation, the follicle remains luteinised, secreting oestrogen and now also progesterone, reverting back to negative feedback on the HPG axis. This, together with inhibin (inhibits FSH) stalls the cycle in anticipation of fertilisation.
3. Luteal Phase
The corpus luteum is the tissue in the ovary that forms at the site of a ruptured follicle following ovulation. It produces oestrogens, progesterone and inhibin to maintain conditions for fertilisation and implantation. At the end of the cycle, in the absence of fertilisation, the corpus luteum spontaneously regresses after 14 days. There is a significant fall in hormones, relieving negative feedback, resetting the HPG axis ready to begin the cycle again.
The uterine ‘secretory phase’ runs alongside the luteal phase. Progesterone stimulates further thickening of the endometrium into a glandular secretory form, thickening of the myometrium, reduction of motility of the myometrium, thick acidic cervical mucus production (a hostile environment to prevent polyspermy), changes in mammary tissue and other metabolic changes.
ANTIPROGESTOGENS
Mifepristone is a partial agonist at progesterone receptors (note: AMH states progesterone receptor antagonist). It dilates the cervix and sensitises the uterus (myometrium) to the action of prostaglandins. Mifepristone is used, in combination with a prostaglandin (e.g. misoprostol), as a medical alternative to surgical termination of pregnancy.
HORMONE REPLACEMENT THERAPY (HRT)
HRT normally involves the cyclic or continuous administration of low doses of one or more estrogens, with or without a progestogen. Estrogen-only HRT is recommended in women post-hysterectomy. Combined HRT is recommended in women with a uterus because of the increased risk of endometrial hyperplasia and cancer associated with estrogen-only HRT. Estrogen relieves symptoms (eg hot flushes, night sweats and urogenital atrophy), caused by reduced endogenous estradiol production. Progestogen reduces risk of endometrial cancer associated with unopposed estrogen.
ORAL CONTRACEPTIVES
Oral contraceptives can either contain both an estrogen and progestogen (Combined Oral Contraceptive aka COC) or contain progestogen only in the pill (aka POP or the mini pill).
COCs inhibit ovulation, reduce receptivity of endometrium to implantation and thicken cervical mucus to form a barrier to sperm. The estrogen inhibits FSH release via negative feedback to inhibit follicle development. The progestogen inhibits the LH surge to prevent ovulation, and thickens cervical mucus to reduce chances of fertilisation.
POPs thicken cervical mucus to impede the passage of sperm and change the endometrium, reducing the potential for implantation. They act on the hypothalamus and suppress pituitary LH surge and may inhibit ovulation. Depot injection and implant reliably suppress ovulation; oral progestogen-only contraceptives suppress ovulation to varying extents (eg levonorgestrel suppresses ovulation in up to 60% of cycles, drospirenone in up to 100% of cycles). Progestogens also induce atrophy within ectopic endometrium.
DRUGS FOR INFERTILITY
Treatment of infertility is highly specialised and depends on the cause. Anovulatory infertility is usually treated first line with letrozole or clomifene. Clomifene (also known as clomiphene), competitively antagonises estrogen receptors in the hypothalamus. This interferes with normal negative feedback mechanisms and increases the release of pituitary gonadotrophins, especially LH, inducing ovulation.
📺 Watch the brief vodcast on Female Hormones and anti-oestrogen, anti-progesterone (13 minutes)
Contraceptives, HRT/PHT
Menopause marks the end of a woman’s reproductive life and is characterised by changes to fertility, hormone release (FSH, LH, progesterone and most importantly oestrogen) and menstrual irregularities before the final menstrual period (FMP).
Menopause occurs as the gonads become less responsive to GnRH. There is a gradual rise in gonadotropins, decreased release of inhibin (inhibits the release of FSH), decreased oestrogen and progesterone release and finally changes in menstrual cycle ultimately resulting in decreased fertility. The normal age of onset for menopause is between 45 and 55 years of age with an average age of 51 years.
Common symptoms include vasomotor symptoms (hot flushes, night sweats, formication), vaginal dryness, atrophic vaginitis, loss or libido and dyspareunia, osteoporosis, weight gain and changes to urinary health (plus more). HRT (hormone replacement therapy) – the most common therapy for menopause aims to reduce the symptoms of menopause (mainly the vasomotor symptoms) and reduce the risk of osteoporosis during menopause and the few years after while on treatment. Therefore the major indications for HRT are the management of physical symptoms of menopause, osteoporosis in women younger than 60 years of age (and early or premature menopause).
The symptoms of menopause mainly caused by the changes in oestrogen production (less oestrogen). Therefore the therapy aims at replacing oestrogen safely to manage the vasomotor symptoms, osteoporosis and other symptoms including sleep disturbances, depression, mood swings, tiredness and poor concentration. The choice of HRT is dependent upon:
- Time since last period
- Whether the woman has an intact uterus
- Symptoms experienced
- Other medical conditions
- Previous response to HRT.
Treatment for menopause includes lifestyle changes – smoking cessation, reduce alcohol, weight reduction, regular exercise and avoidance of symptom triggers. Commonly, women with an intact uterus would be commenced upon a combined oestrogen and progesterone replacement therapy. Women who have had a hysterectomy can be treated with oestrogen. The use of unopposed oestrogen in women with an intact uterus increases the risk of endometriosis and cancer. This is an important treatment differentiator.
The other important treatment differentiator is the time since the last menstrual period. Depending on the duration since LMP, women with an intact uterus may be given either:
- Continuous oestrogen plus cyclical progestin if less than 2 years since their LMP; or
- Continuous oestrogen plus continuous progestin if their LMP was 12-24 months ago, they have had 12 months of continuous oestrogen plus cyclical progestin and their withdrawal bleed is getting lighter.
For women who have had a hysterectomy – they are often commenced on continuous oestrogen supplementation. While in both groups of women oestrogen dose would be titrated to the lowest effective dose – changing the oestrogen, progestin or dosage form can alleviate non-responsive symptoms such as for example heavy bleeding, migraine, vasomotor symptoms (plus others). Thought should also be given to contraceptive protection during the menopause as while the fertility of the woman is declining, there is the possibility of pregnancy. Contraception is required for two years after the FMP if the woman if less than 50 years of age or for one year after the FMP if the woman is 50 years or older.
There are a number of alternative delivery methods useful in HRT and there are also a number of alternative non-hormonal therapies. These alternatives offer some advantage in some presentations and are important strategies to manage specific symptoms.
📺 Watch the brief vodcast on contraceptives and PHT/HRT (17 minutes)
📓 Lecture Notes:
References
Ritter, James M., et al. Rang and Dale’s Pharmacology E-Book, Elsevier, 2018. ProQuest Ebook Central, http://ebookcentral.proquest.com/lib/jcu/detail.action?docID=5651288
https://teachmephysiology.com/reproductive-system/development-maturation/menstrual-cycle/
AMH online
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING
This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.