Fluid, Electrolyte and Acid-Base
Amy McCrystal and Leisa Sanderson
Learning Outcomes
In this chapter, you will learn how to:
- Identify the major compartments of body fluids and their composition
- Explain the physiological mechanisms that govern fluid movement within the body, including the roles of hydrostatic pressure, oncotic pressure, osmosis and active transport
- Analyse how alterations in fluid movement contribute to clinical conditions such as oedema, dehydration and fluid volume imbalances
- Differentiate between hypervolemia and hypovolemia by identifying their causes, risk factors, clinical manifestations and appropriate nursing interventions to manage fluid imbalance and prevent complications
- Describe the normal ranges, physiological functions, causes, signs and symptoms, and nursing interventions for imbalances of key electrolytes, including sodium, potassium, calcium, magnesium, and phosphorus
- Interpret arterial blood gas results to identify and differentiate between respiratory and metabolic acid-base imbalances—acidosis or alkalosis—and determine their compensation status, in order to guide appropriate nursing intervention
- Perform a comprehensive intravenous (IV) therapy assessment by identifying and documenting relevant subjective and objective data, and recognising age-specific considerations for paediatric and older adult populations
- Evaluate the integrity and safety of the entire IV infusion system in accordance with current standards and facility policy.
- Identify, assess, and initiate appropriate interventions for common local complications of peripheral IV therapy—such as phlebitis, infiltration, extravasation, haemorrhage, local infection, and nerve injury—in accordance with clinical guidelines.
Basic Fluid and Electrolyte Concepts
The human body maintains a delicate balance of fluids and electrolytes to help ensure proper functioning and homeostasis. When fluids or electrolytes become imbalanced, individuals are at risk for organ system dysfunction. Nurses must be able to recognise subtle changes in fluid or electrolyte balances in their patients so they can intervene promptly. Timely assessment and intervention prevent complications and save lives.
Before learning about how to care for patients with fluid and electrolyte imbalances, it is important to understand the physiological processes of the body’s regulatory mechanisms. The body is in a constant state of change as fluids and electrolytes are shifted in and out of cells within the body in an attempt to maintain a nearly perfect balance. A slight change in either direction can have significant consequences on various body systems.
Body Fluids
Body fluids consist of water, electrolytes, blood plasma and component cells, proteins, and other soluble particles called solutes. Body fluids are found in two main areas of the body called intracellular and extracellular compartments (Figure 1).
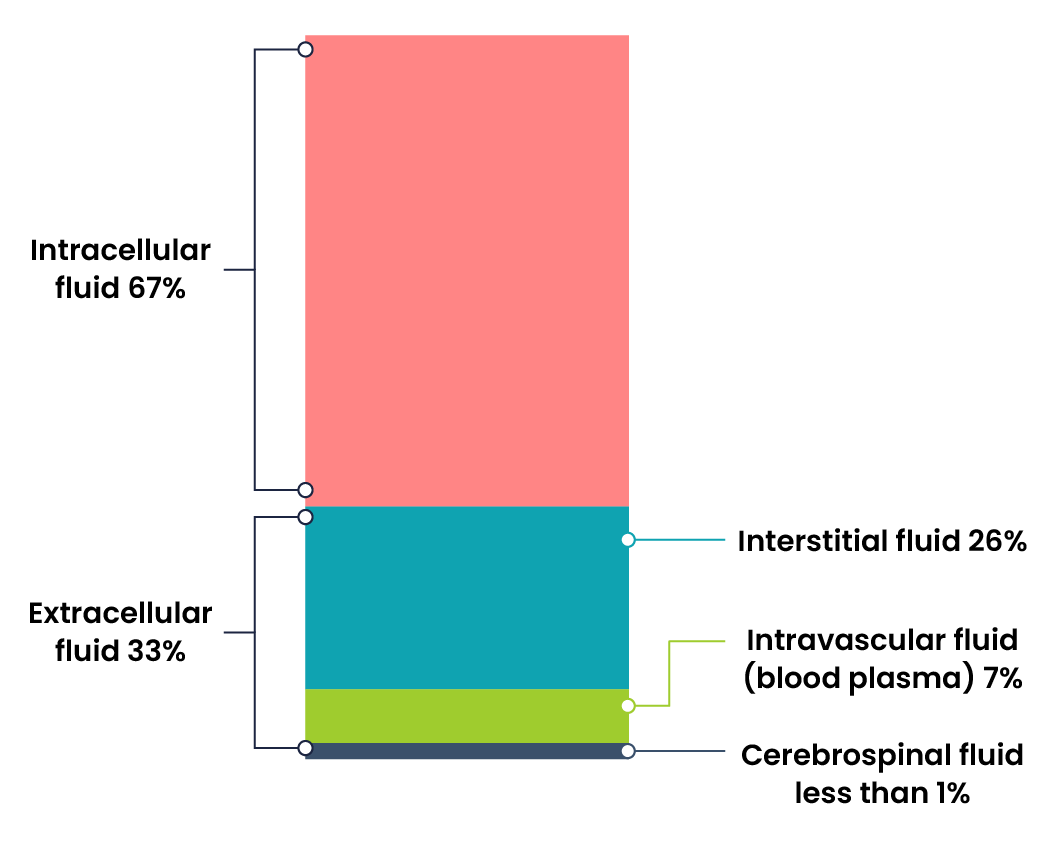
Intracellular Fluids (ICF) are found inside cells and are made up of protein, water, electrolytes, and solutes. The most abundant electrolyte in intracellular fluid is potassium. Intracellular fluids are crucial to the body’s functioning and accounts for 60% of the volume of body fluids and 40% of a person’s total body weight (Hryciw & Bower, 2019).
Extracellular Fluids (ECF) are fluids found outside of cells. The most abundant electrolyte in extracellular fluid is sodium. The body regulates sodium levels to control the movement of water into and out of the extracellular space due to osmosis.
Fluid Movement
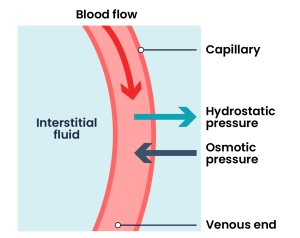
Fluid movement occurs inside the body due to osmotic pressure, hydrostatic pressure, and osmosis. Proper fluid movement depends on intact and properly functioning vascular tissue lining, normal levels of protein content within the blood, and adequate hydrostatic pressures inside the blood vessels. Intact vascular tissue lining prevents fluid from leaking out of the blood vessels. Protein content of the blood (in the form of albumin) causes oncotic pressure that holds water inside the vascular compartment. For example, patients with decreased protein levels (i.e., low serum albumin) experience oedema due to the leakage of intravascular fluid into interstitial areas because of decreased oncotic pressure.
Hydrostatic pressure is defined as pressure that a contained fluid exerts on what is confining it. In the intravascular fluid compartment, hydrostatic pressure is the pressure exerted by blood against the capillaries. Hydrostatic pressure opposes oncotic pressure at the arterial end of capillaries, where it pushes fluid and solutes out into the interstitial compartment. On the venous end of the capillary, hydrostatic pressure is reduced, which allows oncotic pressure to pull fluids and solutes back into the capillary (Brinkman et al., 2023).
Filtration occurs when hydrostatic pressure pushes fluids and solutes through a permeable membrane so they can be excreted. An example of this process is fluid and waste filtration through the glomerular capillaries in the kidneys. This filtration process within the kidneys allows excess fluid and waste products to be excreted from the body in the form of urine.
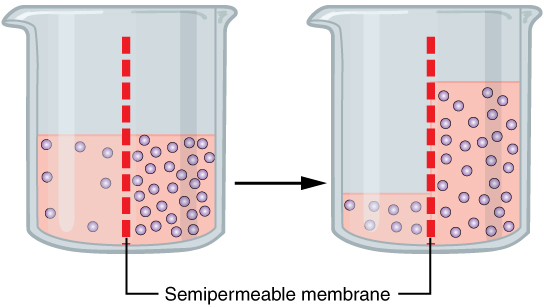
Fluid movement is also controlled through osmosis. Osmosis is water movement through a semipermeable membrane, from an area of lesser solute concentration to an area of greater solute concentration, in an attempt to equalise the solute concentrations on either side of the membrane. Passive transport is when osmosis causes fluid to travel due to a concentration gradient and no energy is expended during the process (Marieb & Hoehn, 2023). Figure 3 demonstrates osmosis, where water has moved to the right side of the membrane to equalise the concentration of solutes on that side with the left side.
Active transport, unlike diffusion, involves moving solutes and ions across a cell membrane from an area of lower concentration to an area of higher concentration. Because active transport moves solutes against a concentration gradient to prevent an overaccumulation of solutes in an area, energy is required for this process to take place.
Fluid and Electrolyte Regulation
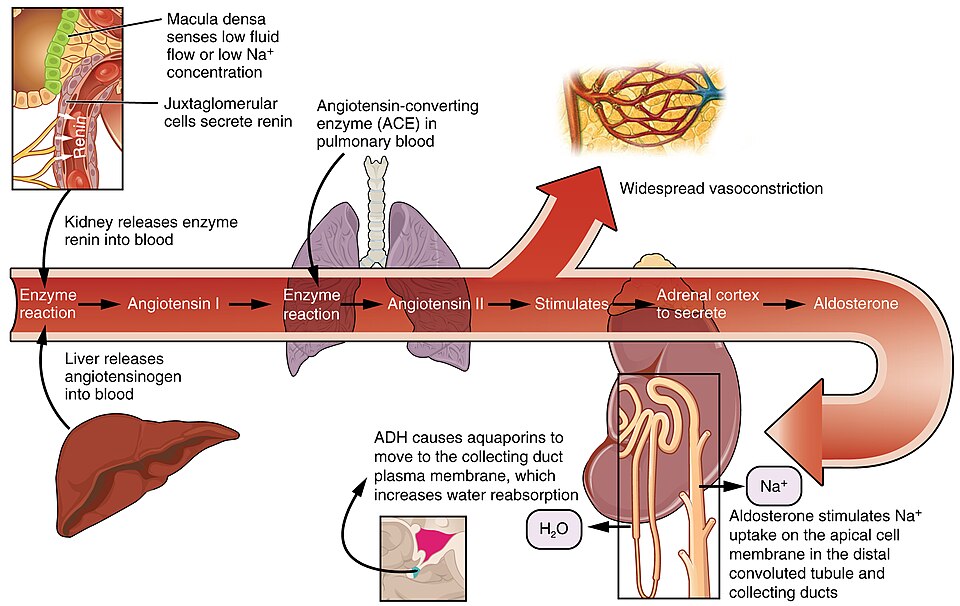
The body must carefully regulate intravascular fluid accumulation and excretion to prevent fluid volume excesses or deficits and maintain adequate blood pressure. Water balance is regulated by several mechanisms, including antidiuretic hormone (ADH), thirst, and the Renin-Angiotensin-Aldosterone System (RAAS).
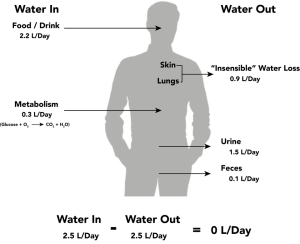
Fluid intake is regulated by thirst. As fluid is lost and the sodium level increases in the intravascular space, serum osmolality increases. Serum osmolality is a measure of the concentration of dissolved solutes in the blood. Osmoreceptors in the hypothalamus sense increased serum osmolarity levels and trigger the release of ADH (antidiuretic hormone) in the kidneys to retain fluid. The osmoreceptors also produce the feeling of thirst to stimulate increased fluid intake. However, individuals must be able to mentally and physically respond to thirst signals to increase their oral intake. They must be alert, fluids must be accessible, and the person must be strong enough to reach for fluids. When a person is unable to respond to thirst signals, dehydration occurs. The average adult intake of fluids is about 2,500 mL per day from both food and drink. An increased amount of fluids is needed if the person has other medical conditions causing excessive fluid loss, such as sweating, fever, vomiting, diarrhea, and bleeding.
The Renin-Angiotensin-Aldosterone System (RAAS) plays an important role in regulating fluid output and blood pressure. When there is decreased blood pressure (which can be caused by fluid loss), specialised kidney cells make and secrete renin into the bloodstream. Renin acts on angiotensinogen released by the liver and converts it to angiotensin I, which is then converted to angiotensin II. Angiotensin II does a few important things. First, angiotensin II causes vasoconstriction to increase blood flow to vital organs. It also stimulates the adrenal cortex to release aldosterone. Aldosterone is a steroid hormone that triggers increased sodium reabsorption by the kidneys and subsequent increased serum osmolality in the bloodstream (Fountain, 2023).
Fluid output occurs mostly through the kidneys in the form of urine. Fluid is also lost through the skin as perspiration, through the gastrointestinal tract in the form of stool, and through the lungs during respiration. 40% of daily fluid output occurs due to these “insensible losses” through the skin, gastrointestinal tract, and lungs and cannot be measured. The remaining 60% of daily fluid output is in the form of urine. Normally, the kidneys produce about 1,500 mL of urine per day when fluid intake is adequate or more accurately 0.5mL/kg/hr. Decreased urine production is an early sign of dehydration or kidney dysfunction. It is important for nurses to assess urine output in patients at risk. If a patient demonstrates less than 30 mL/hour (or 0.5 mL/kg/hour) of urine output prompt action should be taken.
Fluid Imbalance
Two types of fluid imbalances are Fluid volume Excess (also referred to as hypervolemia) and Fluid volume Deficit (also referred to as hypovolemia). These imbalances primarily refer to imbalances in the extracellular compartment but can cause fluid movement in the intracellular compartments based on the sodium level of the blood.
Fluid Volume Excess
Fluid volume excess refers to the abnormal retention of water and sodium in the intravascular compartment, resulting in an isotonic fluid imbalance. This condition may arise from excessive fluid intake, impaired fluid excretion (as seen in heart failure, renal failure, or cirrhosis), or a fluid shift from the interstitial to the plasma space. Individuals at increased risk include those with chronic cardiac, renal, or hepatic conditions, as well as pregnant patients.
Clinical manifestations of fluid volume excess include pitting oedema, ascites, dyspnoea, and crackles on auscultation due to pulmonary congestion. Additional signs may include headache, confusion, lethargy, muscle spasms, jugular vein distension, and rapid weight gain, which is one of the most consistent indicators of fluid overload (Rateau & Hughes, 2023).
Management focuses on treating the underlying cause and may involve fluid and sodium restriction, along with the use of diuretics to promote fluid elimination.
Fluid Volume Deficit
Fluid volume deficit refers to the loss of both water and electrolytes from the extracellular fluid (ECF) in approximately equal proportions, classifying it as an isotonic imbalance. This condition can result from abnormal fluid losses through the gastrointestinal tract, such as vomiting, diarrhoea, or fistula drainage. It may also occur due to plasma or whole blood loss from haemorrhage or burns, or from excessive fluid loss through perspiration, fever, diuretic use, polyuria, or inadequate fluid intake. Additionally, a fluid shift from the intravascular to the interstitial space can contribute to fluid volume deficit (Rateau & Hughes, 2023; Willetts & Schmidt, 2021).
Although often used interchangeably, fluid volume deficit and dehydration are not the same. Dehydration specifically refers to the loss of pure water without a corresponding loss of sodium, whereas fluid volume deficit involves a combined loss of water and electrolytes.
In cases of fluid volume deficit, fluid is initially lost from the intravascular compartment, leading to a condition commonly referred to as hypovolaemia (Quinney et al., 2022). This reduction in circulating blood volume can significantly impair tissue perfusion and organ function if not promptly recognised and treated.
Dehydration
Dehydration, also referred to as a hyperosmolar imbalance, occurs when the body loses water without a corresponding loss of sodium, leading to elevated serum sodium and osmolarity. As water is lost and sodium is retained, fluid shifts from the intracellular and interstitial compartments into the vascular space, resulting in cellular dehydration (Quinney et al., 2022).
Certain populations are at increased risk of dehydration. Older adults are particularly vulnerable due to a reduced thirst sensation, which may delay fluid intake. Other high-risk groups include infants and children, individuals with chronic conditions such as diabetes mellitus or kidney disease, those taking diuretics or medications that increase urine output, and people who exercise or work in hot environments.
Dehydration may also occur in individuals who are hyperventilating, experiencing prolonged fever, in diabetic ketoacidosis, or receiving enteral nutrition without adequate water supplementation (Quinney et al., 2022).
 |
In adults, symptoms of dehydration are as follows:
|
 |
In infants and young children, additional symptoms of dehydration include the following:
|
Electrolytes
Electrolytes play an important role in bodily functions and fluid regulation. There is a very narrow target range for normal electrolyte values, and slight abnormalities can have devastating consequences. For this reason, it is crucial to understand normal electrolyte ranges, causes of electrolyte imbalances, signs and symptoms of imbalances, and appropriate treatments. Click on the plus + sign to view more information on these important electrolytes.
Sodium
Sodium levels in the blood typically range from 135-145 mmol/L (Quinney et al., 2022). Sodium is the most abundant electrolyte in the extracellular fluid (ECF) and is maintained by the sodium-potassium pump. Sodium plays an important role in maintaining adequate fluid balance in the intravascular and interstitial spaces.
Potassium
Potassium levels normally range from 3.5 to 5.0 mmol/L (Quinney et al., 2022). Potassium is the most abundant electrolyte in intracellular fluid and is maintained inside the cell by the sodium-potassium pump. Potassium is regulated by aldosterone in the kidneys and is obtained in the diet through the consumption of foods such as bananas, oranges, and tomatoes. Potassium is necessary for normal cardiac function, neural function, and muscle contractility, including effective contractility of the cardiac muscles. Abnormal potassium levels can cause significantly abnormal heart rhythms and contractility. Potassium is poorly conserved by the body, and much is lost with urine output. For this reason, it is often necessary to provide potassium supplements when administering loop and thiazide diuretics because potassium is excreted from the kidneys along with water (Rateau & Hughes, 2023).
Calcium
Calcium levels normally range from 2.1-2.6 mmol/L (Quinney et al., 2022). Calcium circulates in the bloodstream, but the majority is stored in bones. Calcium is important for bone and teeth structure, nerve transmission, myocardial contraction and muscle contraction. Calcium excretion and reabsorption are regulated by the parathyroid hormone (PTH) that is secreted from the parathyroid glands near the thyroid.
Phosphorus
Phosphorus levels typically range from 0.8 – 1.5mmol/L (Quinney et al., 2022). Phosphorus is stored in the bones and is predominantly found in the ICF with small amounts in the ECF. Phosphorus is important in energy metabolism, RNA and DNA formation, nerve function, muscle contraction, and for bone, teeth, and membrane building and repair. Phosphorus is excreted by the kidneys and absorbed by the intestines. Dietary phosphorus sources include dairy products, red meat, fish, poultry rice and oats (Willetts & Schmidt, 2021).
Magnesium
Magnesium levels typically range from 0.8-1.0 mmol/L (Quinney et al., 2022). Magnesium plays a role in many enzyme systems, including those responsible for carbohydrate metabolism, blood glucose control and blood pressure regulation. Magnesium is essential for normal cardiac, nerve, muscle, and immune system functioning. Around 50-60% of the body’s magnesium is stored in muscle and bones. Only 1% is stored in ECF, with the remainder found in ICF (Rateau & Hughes, 2024).
Acid-Base Balance
As with electrolytes, a correct balance of acids and bases in the body is essential to proper body functioning. Even a slight variance outside of normal can be life-threatening, so it is important to understand normal acid-base values, as well as their causes and how to correct them. The kidneys and lungs work together to correct slight imbalances as they occur. As a result, the kidneys compensate for shortcomings of the lungs, and the lungs compensate for shortcomings of the kidneys.
Arterial Blood Gases
Arterial blood gases (ABG) are measured by collecting blood from an artery, rather than a vein, and are most commonly collected via the radial artery. ABGs measure the pH level of the blood, the partial pressure of arterial oxygen (PaO2), the partial pressure of arterial carbon dioxide (PaCO2), the bicarbonate level (HCO3), and the oxygen saturation level (SaO2).
pH
pH is a scale from 0-14 used to determine the acidity or alkalinity of a substance. A neutral pH is 7, which is the same pH as water. Normally, the blood has a pH between 7.35 and 7.45. A blood pH of less than 7.35 is considered acidic, and a blood pH of more than 7.45 is considered alkaline. The pH of blood is a measure of hydrogen ion concentration. A low pH, less than 7.35, occurs in acidosis when the blood has a high hydrogen ion concentration. A high pH, greater than 7.45, occurs in alkalosis when the blood has a low hydrogen ion concentration. Hydrogen ions are by-products of the metabolism of substances such as proteins, fats, and carbohydrates. These by-products create extra hydrogen ions (H+) in the blood that need to be balanced and kept within normal range as described earlier.
PaCO2
PaCO2 is the partial pressure of arterial carbon dioxide in the blood. The normal PaCO2 level is 35-45 mmHg. CO2 forms an acid in the blood that is regulated by the lungs by changing the rate or depth of respiration. As the respiratory rate increases or becomes deeper, additional CO2 is removed, causing decreased acid (H+) levels in the blood and increased pH (i.e., the blood becomes more alkaline). As the respiratory rate decreases or becomes shallower, less CO2 is removed, causing increased acid (H+) levels in the blood and decreased pH (i.e., the blood becomes more acidic).
HCO3
HCO3 is the bicarbonate level of the blood, and the normal range is 22-26. HCO3 is a base managed by the kidneys and helps to make the blood more alkaline. The kidneys take longer than the lungs to adjust the acidity or alkalinity of the blood, and the response is not visible upon assessment. As the kidneys sense an alteration in pH, they begin to retain or excrete HCO3, depending on what is needed. If the pH becomes acidic, the kidneys retain HCO3 to increase the amount of bases present in the blood to increase the pH (i.e., the blood becomes alkaline). Conversely, if the pH becomes alkalotic, the kidneys excrete more HCO3, causing the pH to decrease (i.e., the blood becomes more acidic).
PaO2
PaO2 is the partial pressure of arterial oxygen in the blood. It more accurately measures a patient’s oxygenation status than SpO2 (the measurement of haemoglobin saturation with oxygen). Therefore, ABG results are also used to manage patients in respiratory distress.
| ABG Component | Description | Adult Normal Value | Critical Value |
| pH |
|
7.35–7.45 | Less than 7.25 Greater than 7.60 |
| PaO2 |
|
80–100 mmHg | Less than 60 mmHg |
| PaCO2 |
|
35–45 mmHg | Less than 25 mmHg Greater than 60 mmHg |
| HCO3 |
|
22–26 mmol/L | Less than 10 mmol/L Greater than 40 mmol/L |
| Less than 88% |
|
95–100% | Less than 88% |
Interpreting Arterial Blood Gases
After the ABG results are received, it is important to understand how to interpret them. A variety of respiratory, metabolic, electrolyte, or circulatory problems can cause acid-base imbalances. Correct interpretation helps the nurse and other health care providers determine the appropriate treatment and evaluate the effectiveness of interventions.
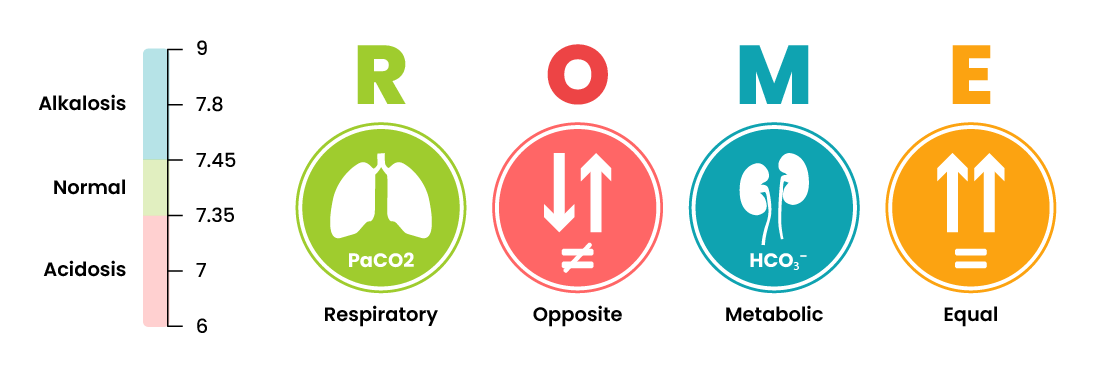
Arterial blood gasses can be interpreted as one of four conditions: respiratory acidosis, respiratory alkalosis, metabolic acidosis, or metabolic alkalosis. Once this interpretation is made, conditions can further be classified as compensated, partially compensated, or uncompensated.
Respiratory Acidosis
Respiratory acidosis develops when carbon dioxide (CO2) builds up in the body (referred to as hypercapnia), causing the blood to become increasingly acidic. Respiratory acidosis is identified when reviewing ABGs and the pH level is below 7.35 and the PaCO2 level is above 45, indicating the cause of the acidosis is respiratory. Respiratory acidosis is typically caused by a medical condition that decreases the exchange of oxygen and carbon dioxide at the alveolar level, such as an acute asthma exacerbation, chronic obstructive pulmonary disease (COPD), or an acute heart failure exacerbation causing pulmonary oedema. It can also be caused by decreased ventilation from anaesthesia, alcohol, or administration of medications such as opioids and sedatives.
Signs of symptoms of hypercapnia vary depending on the level and rate of CO2 accumulation in arterial blood:
Patients with mild to moderate hypercapnia may be anxious and/or complain of mild dyspnoea, daytime sluggishness, headaches, or hypersomnolence.
Patients with higher levels of CO2 or rapidly developing hypercapnia develop delirium, paranoia, depression, confusion, or decreased level of consciousness that can progress to seizures and coma as levels continue to rise.
Treatment for respiratory acidosis typically involves improving ventilation and respiration by removing airway restrictions, reversing oversedation, administering nebuliser treatments, or increasing the rate and depth of respiration by using a BiPAP or CPAP device. BiPAP and CPAP devices provide non-invasive positive pressure ventilation to increase the depth of respirations, remove carbon dioxide, and oxygenate the patient. If these non-invasive interventions are not successful, mechanical ventilation may be necessary (Quinney et al., 2022).
Respiratory Alkalosis
Respiratory alkalosis develops when the body removes too much carbon dioxide through respiration, resulting in increased pH and an alkalotic state. When reviewing ABGs, respiratory alkalosis is identified when pH levels are above 7.45 and the PaCO2 level is below 35. With respiratory alkalosis, notice that as the PaCO2 level decreases, the pH level increases.
Respiratory alkalosis is caused by hyperventilation that can occur due to anxiety, panic attacks, pain, fear, head injuries, or mechanical ventilation. Fever, medication overdoses of salicylates and other toxins can also cause respiratory alkalosis initially and then often progress to metabolic acidosis in later stages (Czarecki et al., 2023).
Patients experiencing respiratory alkalosis often report feelings of shortness of breath, dizziness or light-headedness, chest pain or tightness, paraesthesia, and palpitations as a result of decreased carbon dioxide levels (Czarecki et al., 2023). Respiratory alkalosis is not considered life-threatening, and treatment should focus towards treating the underlying cause of hyperventilation and managing hypoxemia if present. Acute management of patients who are hyperventilating should focus on patient reassurance, an explanation of the symptoms the patient is experiencing, removal of any stressors, and initiation of breathing retraining. Breathing retraining attempts to focus the patient on abdominal (diaphragmatic) breathing.
Metabolic Acidosis
Metabolic acidosis occurs when there is an accumulation of acids (hydrogen ions) and not enough bases (HCO3) in the body. Under normal conditions, the kidneys work to excrete acids through urine and neutralise excess acids by increasing bicarbonate (HCO3) reabsorption from the urine to maintain a normal pH. When the kidneys are not able to perform this buffering function to the level required to excrete and neutralise the excess acid, metabolic acidosis results.
Metabolic acidosis is characterised by a pH level below 7.35 and an HCO3 level below 22 when reviewing ABGs. It is important to notice that both the pH and HCO3 decrease with metabolic acidosis (i.e., the pH and HCO3 move in the same downward direction). A common cause of metabolic acidosis is diabetic ketoacidosis, where acids called ketones build up in the blood when blood sugar is extremely elevated. Another common cause of metabolic acidosis in hospitalised patients is lactic acidosis, which can be caused by impaired tissue oxygenation. Metabolic acidosis can also be caused by increased loss of bicarbonate due to severe diarrhea or from renal disease that causes decreased acid elimination. Additionally, toxins such as salicylate excess can cause metabolic acidosis (Czarecki et al., 2023)
Nurses may first suspect that a patient has metabolic acidosis due to rapid breathing that occurs as the lungs try to remove excess CO2 in an attempt to resolve the acidosis. This rapid breathing is otherwise known as Kussmaul respirations (Czarecki et al., 2023). Other symptoms of metabolic acidosis include altered mental state, weakness, nausea and abdominal pain. Electrolyte imbalances, in particular hyperkalemia, can be severe; it is therefore important to ensure prompt treatment. Treatment will be dependent on the underlying cause. Treatment includes IV fluids to improve hydration status, glucose management, and circulatory support. When pH drops below 7.1, IV sodium bicarbonate is often prescribed to help neutralise the acids in the blood (Czarecki et al., 2023).
Metabolic Alkalosis
Metabolic alkalosis occurs when there is too much bicarbonate (HCO3) in the body or an excessive loss of acid (H+ ions). Metabolic alkalosis is defined by a pH above 7.45 and an HCO3 level above 26 on ABG results. Note that both pH and HCO3 are elevated in metabolic alkalosis.
Metabolic alkalosis can be caused by gastrointestinal loss of hydrogen ions, excessive urine loss, excessive levels of bicarbonate, or a shift of hydrogen ions from the bloodstream into cells. Prolonged vomiting or nasogastric suctioning are amongst the common causative factors. Gastric secretions have high levels of hydrogen ions (H+), so as acid is lost, the pH level of the bloodstream increases (Rateau & Hughes, 2023). Intravenous administration of sodium bicarbonate can also cause metabolic alkalosis due to increased levels of bases introduced into the body.
A nurse may first suspect that a patient has metabolic alkalosis due to a decreased respiratory rate (as the lungs try to retain additional CO2 to increase the acidity of the blood and resolve the alkalosis). The patient may present with dizziness, paraesthesia, numbness and tingling in the extremities (Rateau & Hughes, 2023).
Treatment is prescribed based on the ABG results and the suspected cause. For example, treat the cause of the vomiting, stop the gastrointestinal suctioning, or stop the administration of diuretics and correct volume depletion with an isotonic sodium chloride solution. If hypokalaemia is present, replacement will be required (Rateau & Hughes, 2023).
ABG interpretation
A simple way to remember how to interpret ABGs is by using the ROME method of interpretation, which stands for Respiratory Opposite, Metabolic Equal. This means that the respiratory component (PaCO2) moves in the opposite direction of the pH if the respiratory system is causing the imbalance. If the metabolic system is causing the imbalance, the metabolic component (HCO3) moves in the same direction as the pH.
Intravenous Therapy
Intravenous (IV) fluid and electrolyte therapy is essential for managing a variety of fluid and electrolyte imbalances. Patients may require IV fluids for maintenance, when oral intake is insufficient, or for replacement, when fluid losses have occurred or are ongoing. The type and amount of IV fluid prescribed are based on the patient’s daily maintenance needs and clinical assessment, including pathology results. IV fluids are classified by their tonicity—the concentration of solutes in comparison to plasma—which determines how they affect fluid movement between body compartments. The three main categories are isotonic, hypotonic, and hypertonic solutions.
Solutions for IV Administration
Isotonic solution
Isotonic solutions have a similar concentration of solutes as plasma, with an osmolarity of approximately 250–375 mOsm/L (Rateau & Hughes, 2023). These fluids expand the ECF without causing fluid shifts between compartments, making them ideal for treating ECF volume deficits.
Hypotonic solution
Hypotonic solutions have a lower solute concentration than plasma (<250 mOsm/L), causing water to move into cells (Rateau & Hughes, 2023). These fluids are used to treat cellular dehydration and hypernatremia.
Hypertonic solution
Hypertonic solutions have a higher solute concentration than plasma (>375 mOsm/L) (Rateau & Hughes, 2023), drawing water out of cells into the ECF. These are used to treat hyponatremia and cerebral oedema.
Administering and Management of Intravenous Therapy (IV)
Intravenous Access
Intravenous access is established by inserting a flexible catheter into the venous system for the administration of IV therapy. Access may be gained either peripherally or centrally, depending on several factors, including the duration of therapy, the type of solution or medication to be administered, the condition of the veins, and the overall clinical status of the patient.
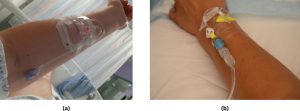
Peripheral Intravenous Catheters (PIVCs)
A peripheral intravenous catheter (PIVC), commonly referred to as a cannula, is the most frequently used type of intravenous access device. Up to 70% of hospitalised patients in Australia require at least one cannula during their admission (ACSQHC, 2021). A cannula is a short, single-lumen catheter inserted into a peripheral vein, typically in the arm, hand, or foot. The size and diameter of the cannula are selected based on the intended therapy.
A PIVC is considered appropriate when therapy is short-term, the patient has suitable peripheral vasculature, and peripheral circulation is adequate (Thompson & Steinheiser, 2024). Infusion of high-osmolarity solutions can irritate peripheral veins, and prolonged administration may damage the vessel wall, potentially leading to complications such as phlebitis, thrombosis, or occlusion (NSW Agency for Clinical Innovation, 2021). Patients with poor peripheral circulation, those requiring long-term therapy (weeks to months), or those with fragile veins should be assessed for alternative vascular access devices.
The most commonly selected sites for PIVC insertion include the metacarpal, basilic, and cephalic veins, due to their accessibility and stability. While the antecubital fossa is often chosen for its ease of insertion, it is generally avoided as a primary site because frequent flexion in this area increases the risk of catheter dislodgement and related complications.
Central Venous Access Devices (CVADs)
A central venous access device (CVAD) is inserted into a large vein and advanced into the superior or inferior vena cava. CVADs may be inserted centrally or peripherally and are classified based on the method of insertion and catheter type.
CVADs offer several advantages over PIVCs, including the ability to administer a wide range of therapies such as chemotherapy, total parenteral nutrition, long-term intravenous medications or IV therapy, and irritant solutions. Their capacity to deliver high-osmolarity substances reduces the risk of complications like phlebitis, thrombosis, and occlusion that may occur with peripheral access (NSW Agency for Clinical Innovation, 2021).
However, patients with a central line are at risk of developing a central line–associated bloodstream infection (CLABSI)—a preventable but potentially life-threatening infection. CLABSIs can result from contamination during insertion, improper maintenance, or secondary infections. Strict protocols govern the insertion, access, and management of all central lines; clinicians must refer to local facility policies for guidance.
There are three primary types of CVADs:
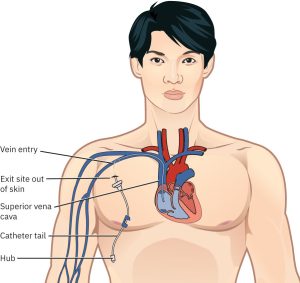
Central Venous Catheter (CVC):
Inserted centrally, typically via the internal jugular, subclavian, or femoral veins. CVCs usually have 2–3 lumens and are sized 7–8 French (Fr). Non-tunnelled CVCs are generally used for up to 10 days (NSW Agency for Clinical Innovation, 2021). These devices carry a higher risk of CLABSI and, in the case of subclavian insertions, an added risk of pneumothorax (Baldauf, 2025).
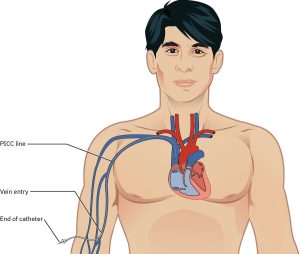
Peripherally Inserted Central Catheter (PICC):
Inserted via a peripheral vein, most commonly the basilic vein (Kehagias et al., 2023). PICCs are smaller (3–6 Fr) and less suitable for rapid fluid resuscitation. They are designed for longer-term use, typically up to three months. While PICCs have a lower risk of CLABSI compared to CVCs, they are associated with a higher risk of catheter-related venous thrombosis (NSW Agency for Clinical Innovation, 2021).
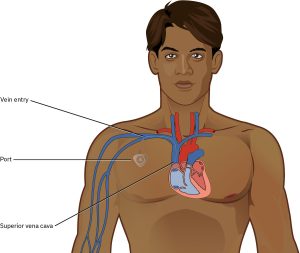
Port-a-Cath (Implantable Port):
A type of tunnelled CVC, usually implanted in the anterior chest near the clavicle. The port is placed in a subcutaneous pocket with no external components. Ports are typically 5–9 Fr in size and may have one or two lumens. They are intended for long-term use, particularly in patients with chronic conditions, and can remain in place for many years (Kehagias et al., 2023). The main disadvantage is the need for percutaneous access using specialised needles, which can be painful and may limit infusion rates.
 |
Safety Alert
|
Peripheral Intravenous Catheter (PIVC) Size, Colours and Flow Rates
Peripheral IV catheters are available in a variety of sizes, most commonly ranging from 14 gauge to 26 gauge. Note that the lower the gauge size, the wider the diameter of the catheter, with 14-gauge catheters allowing for the greatest flow rate. Catheter sizes are colour coded to allow for easy identification of the catheter size after a vein is accessed.
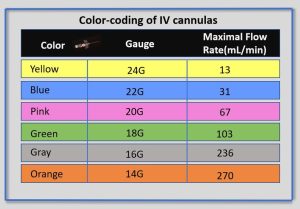
Safety Considerations when Using Intravenous Route
When administering IV therapies, nurses should comply with safety precautions to reduce the risk of potential complications. These complications may be local or systemic and include infection, damage to the vein and surrounding tissue, air emboli, blood clots, and fluid overload. The nurse plays a key role in monitoring for signs of complications, initiating safety interventions to reduce the risk and severity of complications, and performing routine assessments of the IV. For example, replacing electrolytes such as sodium, potassium, or magnesium via the IV route is faster than the oral route; however, the nurse must be mindful of giving the correct amounts of electrolytes and maintaining therapeutic levels. The nurse will monitor the patient’s sodium and potassium levels through blood tests, as well as assess the patient for symptoms of overdose or toxicity.
Infection Control in IV Therapy
Infection control is essential when preparing and administering intravenous (IV) fluids and medications. Using aseptic technique helps prevent the introduction of harmful microorganisms into the bloodstream.
Key principles:
- Hand hygiene must be performed before handling any IV equipment, medications, or fluids.
- Prepare IV therapies in a clean area on a clean surface.
- Use single-use equipment (e.g., syringes, needles, IV sets) for one patient only.
- Open packaging as it is designed, do not rip, tear or ‘pop’ open packages.
- Disinfect all access points (e.g., hubs, ports, connectors) with a 70% alcohol wipe before use. Scrub for 5–15 seconds and allow to dry completely (about 5 seconds for alcohol, 20 seconds for chlorhexidine-alcohol) (NHMRC, 2019).
- Avoid disconnecting IV lines unless necessary. If disconnected, replace the administration set.
- Disinfect vial stoppers before inserting a needle.
- Administration set must not be disconnected short-term and then reconnected. If disconnection is required, a new administration set must be used.
(Nickel et al., 2024)
Monitoring for Potential Complications
Several potential complications may arise from peripheral intravenous therapy. It is the responsibility of the nurse to prevent, assess, and manage signs and symptoms of complications. Complications can be categorised as local or systemic.
IV Administration Equipment
Intravenous (IV) substances are administered through flexible plastic tubing called an IV administration set. The IV administration set connects the bag of solution to the patient’s IV access site. The set may be considered primary or secondary. The administration sets are designed to regulate fluid delivery, ensuring that you administer the correct amount at the correct rate. Failure to regulate IV flow rate appropriately can cause complications from receiving too much fluid too quickly or not enough fluid fast enough. There are several different ways of regulating IV flow rates, including gravity infusion, pump infusion, IV push, continuous single infusions, and continuous multiple infusions.
Primary Administration Sets
Primary IV administration sets are designed as the main fluid delivery device and consist of the following parts:
- Sterile spike: Used to spike the IV fluid bag and must be kept sterile.
- Roller clamp: Used to regulate the speed or stop an infusion by gravity.
- Drip chamber: Allows air to rise out from a fluid so that it is not passed onto the patient. The drip chamber should be kept ¼ to ½ full of solution. When setting a rate by gravity to “drops per minute,” the dripping from this chamber is counted.
- Backcheck valve: Prevents fluid or medication from travelling up into the primary IV bag.
- Access ports: Used to infuse secondary medications and to administer IV push medications. These may also be referred to as “Y ports.”
Secondary Administration Sets
Secondary IV administration sets are used to intermittently administer a secondary medication, such as an antibiotic, while the primary IV is also running. Secondary IV tubing is shorter in length than primary tubing and is connected to a primary line via an access port above the infusion pump. The infusion pump is then set at the prescribed secondary infusion rate while the secondary medication is administered. By hanging the secondary medication bag higher than the primary bag, gravity pulls fluid from the secondary bag until it is empty rather than the primary bag.
Secondary medications may be “piggybacked” into primary infusion lines so the solution from the primary fluid line can be used to prime the secondary tubing. To prime the secondary tubing, after the secondary tubing is connected to the primary tubing, the bag connected to the secondary tubing is held lower than the primary bag, causing fluid from the primary tubing to backflow up the secondary tubing. This eliminates air from the secondary tubing.
See Figure 12 for an illustration of the setup of primary and secondary administration sets for primary administration of fluids and secondary administration of medication by gravity.

Priming IV Tubing
Priming refers to the process of filling the IV tubing with IV solution prior to attaching it to the patient. Primary administration sets, secondary administration sets, and extension tubing must be primed with IV solution to prevent air from entering the patient’s circulatory system and causing an air embolism.
Gravity flow Infusion
When infusing IV fluids by gravity, the nurse regulates the infusion rate by using a roller clamp on the IV tubing, which can either speed up or slow down the flow of IV fluids. An IV flow rate for gravity is calculated in drops per minute (DPM). Common drop factor rates for IV administration set in Australia include 15 DPM, 20 DPM and 60DPM. To calculate the DPM, multiply the infusion rate (mL/hr) by the IV drop factor found on the IV tubing, then divide by the infusion time in minutes. For example, a fluid that is to be infused at a rate of 100mL/hr via 20 DPM tubing would infuse at a rate of 33 drop/min [(100 × 20)/60 = 33.33 drop/min]. Drops can only be administered whole, so round up if greater than 0.5, and round down if less than 0.5.
Electronic Infusion Pumps
Electronic infusion pumps regulate the delivery of intravenous fluids and medications by controlling the infusion rate in millilitres per hour (mL/hr). Their use is recommended across all clinical settings to minimise infusion-related medication errors (Nickel, 2024). Smart pumps, or Dose Error Reduction Systems (DERS), incorporate drug libraries with predefined “soft” and “hard” limits for medication dosing and infusion rates. These systems are designed to enhance safety by preventing errors in medication selection, dosing, and administration. Nurses are responsible for selecting the correct medication, dose, and infusion duration to ensure safe and accurate delivery.

IV Push Administration
Some medications may require administration via IV push, typically delivered through the access port closest to the patient on the primary IV tubing. If the patient is not receiving continuous IV fluids, the medication is administered directly via the cannula, with the site flushed both before and after administration to maintain patency and ensure complete delivery.
The method of administration should always align with facility policy and be guided by evidence-based resources such as the Australian Injectable Drugs Handbook. For further guidance on IV medication administration, refer to the Parenteral Medication Administration chapter.

|
|
In accordance with the National Standard for User-Applied Labelling of Injectable Medicines, all IV fluid lines must be clearly labelled to reduce the risk of patient harm. Implementation of the Labelling Standard is a mandatory requirement for meeting the National Safety and Quality Health Service (NSQHS) Standards – in particular, NSQHS Standard 4: Medication Safety, and NSQHS Standard 5: Patient Identification and Procedure Matching (ACSQHC, 2015). The Labelling Standard ensures that healthcare professionals can accurately identify the medicine or fluid, the route of administration, and the intended patient. This standard promotes safer use of injectable medicines by requiring consistent label format, content, and placement. All containers and conduits (e.g. IV lines) that no longer display original packaging must be labelled before leaving the hands of the person preparing the medicine. Unlabelled medicines or fluids are considered unsafe and must be discarded. Proper labelling is a critical role of the registered nurse in preventing administration errors such as wrong medicine, dose, route, or patient (ACSQHC, 2015). Further information on the labelling of IV medications and fluid can be found on the National Standard for User-applied Labelling of Injectable Medicines, Fluids and Lines webpage.
|
Administration of Intravenous Fluid via PIVC
Life Span Considerations
When caring for PIVC or delivering IVT, specific considerations are necessary for children and older adults due to their unique physiological characteristics and potential vulnerabilities.
 |
Infants and Children
Due to their fragile skin, small veins, limited ability to communicate discomfort, and inability to understand safety aspects, extra caution is required (Nickel et al., 2024). Consider using volume-controlled infusion sets and locked infusion pumps to prevent interference or accidental changes in flow rate. Needleless systems and secure dressings help reduce the risk of dislodgement, while protective devices may prevent tampering. Regular inspection, at least hourly during infusions, is essential to detect early signs of infiltration or extravasation. During insertion or removal of the PIVC consider age-appropriate distraction techniques, comfort positioning, involving parents, and applying pain reduction strategies such as the application of Elma cream (Thompson et al., 2024). Joint stabilisation may be required for PIVC security but should be used cautiously, ensuring it does not compromise circulation or skin integrity (Nickel et al., 2024). |
|
|
Older Adults Older adults experience age-related changes in skin and vasculature, such as reduced dermal thickness and vein fragility, increasing the risk of infiltration, extravasation, and phlebitis (Thompson et al., 2024). Frequent checks of the insertion site are necessary to detect complications early. Assess for any deficits in cognitive ability, dexterity, and communication (e.g., changes in vision, hearing, speech) that may impact understanding and management of IV therapy (Nickel et al., 2024). Older adults may experience difficulties manipulating additional devices such as IV poles. Develop a plan of care to accommodate additional assistance with activities of daily living if required. Considerations also include altered immune response, drug metabolism, and volume tolerance, which may affect device selection and infusion parameters. The nurse should tailor IV therapy to accommodate these physiological changes and monitor for side effects and therapeutic response (Nickel et al., 2024). IV flow rates and fluid balance must be monitored closely to avoid fluid overload.
|
Key Takeaways
In this chapter, we covered:
- Understanding body fluid compartments and the physiological mechanisms that regulate fluid movement—such as hydrostatic pressure, oncotic pressure, osmosis, and active transport—is essential for identifying and managing fluid imbalances like oedema, dehydration, hypervolemia, and hypovolemia.
- Each fluid imbalance has specific causes, risk factors, and clinical manifestations, requiring appropriate nursing interventions to prevent complications.
- Electrolytes such as sodium, potassium, calcium, magnesium, and phosphorus play critical roles in nerve, muscle, and cardiac function, and their imbalances can lead to serious health issues. Nurses must recognise normal ranges, symptoms, and treatment strategies for these imbalances.
- Accurate interpretation of arterial blood gases using the ROME method enables differentiation between respiratory and metabolic acidosis or alkalosis and determines the compensation status, guiding timely and effective nursing care.
- A thorough IV therapy assessment requires the nurse to gather both subjective and objective data, taking into account patient-specific risks such as allergies, chronic conditions, and age-related factors. Regular, age-appropriate monitoring of the IV site and infusion system is essential to prevent complications and ensure safe, effective treatment.
- Early recognition and prompt intervention are critical in preventing and managing complications of peripheral IV therapy. Nurses must routinely assess IV sites for signs of local issues like phlebitis, infiltration, or infection and respond immediately to reduce patient harm and prevent progression to systemic complications.
References
Alamer, F., & Alanazi, A. T. (2023). The impact of smart pump technology in the healthcare system: A scope review. Cureus, 15(3), Article e36007. https://doi.org/10.7759/cureus.36007
Australian Commission on Safety and Quality in Health Care. (2015). National standard for user-applied labelling of injectable medicines, fluids and lines. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/national-standard-user-applied-labelling-injectable-medicines-fluids-and-lines
Australian Commission on Safety and Quality in Health Care. (2021). Management of peripheral intravenous catheters: Clinical care standard. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/management-peripheral-intravenous-catheters-clinical-care-standard-2021
Baldauf, B., Cemin, R., Hummel, J., Bonnemeier, H., & Assadian, O. (2025). Central vascular access devices: Current standards and future implications. Journal of Vascular Diseases, 4(1), 3. https://doi.org/10.3390/jvd4010003
Brinkman, J. E., Dorius, B., Sharma, S. (2023, January 27). Physiology, body fluids. In M. Abdelsattar, L. T. Abernethy, W. B. Ackley, T. S. Adolphe, T. C. Aeby, S. Agadi, P. Agasthi, R. Agnihotri, S. Ahmad, A. Ahmed, I. Ahmed, R. A. Ahmed, A. M. Akanmode, R. Akella, Y. Al Ghabra, Y. Al Khalili, E. Al Zaabi, M. H. Alahmadi, G. Alexander, … H. Zulfiqar (Eds). StatPearls. StatPearls Publishing. Retrieved June 25, 2025, from https://www.ncbi.nlm.nih.gov/books/NBK482447/
Czarnecki, P. G., Rhee, C., Owyang, C. G., & Renne, B. C. (2023). Acid–base interpretation. In K. Shah & J. Lee (Eds.), Practical emergency resuscitation and critical care (pp. 319–332). Cambridge University Press.
Emmett, M., & Szerlip, H. (2024a). Approach to the adult with metabolic acidosis. UpToDate. Retrieved June 24, 2025, from https://www.uptodate.com/contents/approach-to-the-adult-with-metabolic-acidosis
Emmett, M., & Szerlip, H. (2024b). Causes of metabolic alkalosis. UpToDate. Retrieved June 24, 2025, from https://www.uptodate.com/contents/causes-of-metabolic-alkalosis
Fountain, J. H., Kaur, J., & Lappin, S. L. (2023). Physiology, renin angiotensin system. In M. Abdelsattar, L. T. Abernethy, W. B. Ackley, T. S. Adolphe, T. C. Aeby, S. Agadi, P. Agasthi, R. Agnihotri, S. Ahmad, A. Ahmed, I. Ahmed, R. A. Ahmed, A. M. Akanmode, R. Akella, Y. Al Ghabra, Y. Al Khalili, E. Al Zaabi, M. H. Alahmadi, G. Alexander, … H. Zulfiqar (Eds). StatPearls. StatPearls Publishing. Retrieved June 24, 2025, from https://www.ncbi.nlm.nih.gov/books/NBK470410/
Gorski, L. A., Hadaway, L., Hagle, M. E., Broadhurst, D., Clare, S., Kleidon, T., Meyer, B. M., Nickel, B., Rowley, S., Sharp, E., & Alexander, M. A. (2021). Infusion therapy standards of practice: 8th edition [Supplemental material]. Journal of Infusion Nursing, 44(1S), S1–S224. https://doi.org/10.1097/NAN.0000000000000396
Kehagias, E., Galanakis, N., & Tsetis, D. (2023). Central venous catheters: Which, when and how. British Journal of Radiology, 96(1151), Article 20220894. https://doi.org/10.1259/bjr.20220894
Hryciw, D., & Bower, A., S. (2019). Fluid, electrolyte and acid-base balance. In J. Craft, C. Gordon, S. E. Huether, K. L. McCance, & V. L. Brashers (Eds.), Understanding pathophysiology ANZ (3rd ed., pp. 882-910). Elsevier.
Marieb, E. N., & Hoehn, K. (2023). Human anatomy & physiology (12th ed., Global ed.). Pearson.
MedlinePlus. (n.d.). Respiratory acidosis. National Library of Medicine. https://medlineplus.gov/ency/article/000092.htm
MedlinePlus. (2023, December 29). Dehydration. National Library of Medicine. https://medlineplus.gov/dehydration.html
National Health and Medical Research Council. (2019). Australian guidelines for the prevention and control of infection in healthcare. Australian Commission on Safety and Quality in Health Care. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/australian-guidelines-prevention-and-control-infection-healthcare
Nickel B., Gorski L., Kleidon T., Kyes, A., DeVries, M., Keogh, S., Meyer, B., Sarver, M. J., Crickman, R., Ong, J., Clare, S., & Hagle, M. E. (2024). Infusion therapy standards of practice: 9th edition [Supplemental material]. Journal of Infusion Nursing, 47(1S), S1-S285. https://doi.org/10.1097/nan.0000000000000532
NSW Agency for Clinical Innovation. (2021). Central venous access devices (CVAD): Clinical practice guide. https://aci.health.nsw.gov.au/__data/assets/pdf_file/0010/239626/ACI-CVAD-clinical-practice-guide.pdf
Quinney, L., Hayes, B., & Carroll, A. (2021). Fluid, electrolyte and acid-base imbalance. In A. Berman, G. Frandsen, S. J. Snyder, T. Levett-Jones, A. Burston, T. Dwyer, M. Hales, N. Harvey, L. Moxham, T. Langtree, K. Reid-Searl, & D. Stanley (Eds.), Kozier and Erb’s fundamentals of nursing (5th Australian ed., Vol. 3, pp. 1512 -1571). Pearson .
Rateau, M. R., & Hughes, J. A. (2023). Nursing management: Fluid, electrolyte and acid-base imbalances. In D. Brown, T. Buckley, R. Aitken, & H. Edwards (Eds.), Lewis’s medical-surgical nursing: Assessment and management of clinical problems (6th ed., Australian and New Zealand ed., pp. 345-377). Elsevier .
Thompson, J., Steinheiser, M. M., Hotchkiss, J. B., Davis, J., DeVries, M., Frate, K., Helm, R., Jungkans, C. W., Kakani, S., Lau, S., Lindell, K., Landrum, K. M., McQuillan, K. A., Shannon, D., Wuerz, L., & Pitts, S. (2024). Standards of care for peripheral intravenous catheters: Evidence-based expert consensus [Supplemental material]. British Journal of Nursing, 33(21), S32–S46. https://doi.org/10.12968/bjon.2024.0422
Willetts, G., & Schmidt, L. (2021). Balancing fluid, electrolyte and acid-base status. In J. Crisp, C. Douglas, G. Rebeiro, & D. Waters (Eds.), Potter and Perry’s fundamentals of nursing (6th Australian and New Zealand ed., pp. 826-911). Elsevier.
Chapter Attribution
This chapter has been adapted in parts from:
Clinical nursing skills (2024) by Christie Bowen et al., OpenStax, is used under a CC BY licence.
Nursing fundamentals 2e (n.d.) by Open Resources for Nursing, Chippewa Valley Technical College, is used under a CC BY licence.
Nursing skills 2e (2023) by Open Resources for Nursing, Chippewa Valley Technical College, is used under a CC BY licence.
Media Attributions
- Intracellular and Extracellular Compartments © Welcome1To1The1Jungle adapted by Eileen Siddins is licensed under a CC BY (Attribution) license
- Capillary Microcirculation Hydrostatic Pressure © Kes47 adapted by Eileen Siddins is licensed under a Public Domain license
- 0307_Osmosis © Openstax is licensed under a CC BY (Attribution) license
- 2626_Renin_Aldosterone_Angiotensin © OpenStax College is licensed under a CC BY (Attribution) license
- Water_balance © David Walsh & Alan Sved is licensed under a CC BY-SA (Attribution ShareAlike) license
- ROME and PH scale © Eileen Siddins is licensed under a CC BY-NC (Attribution NonCommercial) license
- OSX_Skills_13_02_Cannulas © (a) Unknown“Cannula A.jpg” byUnknown on Wikimedia Commons, in the Public Domain. (b), “Saline lock” by Glynda Rees Doyle & Jodie Anita McCutcheon is licensed under a CC BY (Attribution) license
- OSX_Skills_13_02_Nontunnel © Christy Bowen is licensed under a CC BY (Attribution) license
- OSX_Skills_13_02_PICC © Christy Bowen is licensed under a CC BY (Attribution) license
- OSX_Skills_13_02_Port © Christy Bowen is licensed under a CC BY (Attribution) license
- Color-coding_of_IV_cannulas © Dr.Vijaya chandar is licensed under a CC BY-SA (Attribution ShareAlike) license
- 9e608fdaca5ee91761fcd4532204ec3f5af17e4f © Glynda Rees Doyle & Jodie Anita McCutcheon/McCutcheon/British Columbia Institute of Technology is licensed under a CC BY (Attribution) license
- Infusion pump © George Coogan is licensed under a CC BY (Attribution) license
- Flush the saline lock © Glynda Rees Doyle & Jodie Anita McCutcheon/British Columbia Institute of Technology is licensed under a CC BY (Attribution) license
Fluid movement occurs inside the body due to osmotic pressure, hydrostatic pressure, and osmosis. Proper fluid movement depends on intact and properly functioning vascular tissue lining, normal levels of protein content within the blood, and adequate hydrostatic pressures inside the blood vessels. Intact vascular tissue lining prevents fluid from leaking out of the blood vessels. Protein content of the blood (in the form of albumin) causes oncotic pressure that holds water inside the vascular compartment. For example, patients with decreased protein levels (i.e., low serum albumin) experience oedema due to the leakage of intravascular fluid into interstitial areas because of decreased oncotic pressure.


