Parenteral Medication Administration
Penelope Coogan and Leisa Sanderson
Learning Outcomes
In this chapter you will learn how to:
- Identify the different parenteral routes of medication administration
- Describe the nurse’s responsibility in ensuring safe and effective parenteral medication administration
- Recognise the equipment used for parenteral medication administration
- Demonstrate safe practices for administering medications via the intradermal, subcutaneous, intramuscular and intravenous routes to people across all stages of the lifespan
- Document actions and evaluate effectiveness.
Parenteral medication administration refers to injecting medications directly into the tissues and circulatory system, bypassing the skin, mucous membranes, and gastrointestinal tract.
Administering medications via the parenteral route may be useful when the medication is poorly absorbed orally, if the patient is unable to tolerate oral administration or when immediate or delayed onset is required.
This chapter will describe the various parental routes such as intradermal (ID), subcutaneous (Subcut), intramuscular (IM) and intravenous (IV). It will supply you with guidelines, preparing you to administer medications via this route effectively and safely.
Equipment Used for Parenteral Medication Administration
When administering medication by the parenteral route, it is essential to understand the equipment required. The following considers key equipment used, including syringes, needles, needless systems and sharp safety devices. Each has a vital role in ensuring safe, accurate and effective medication delivery while minimising the risk of harm to both the patient and nurse.
Parenteral Medication Formulations
Parenteral medications may be supplied in a variety of packages, such as vials, glass or plastic ampoules, and prefilled cartridges. Sometimes, the medication will come prepared, ready for the nurse to administer. Other times, it may be necessary for the nurse to withdraw a dose of medication from a vial, add a liquid (diluent) to a powdered medication (solute) to form a liquid solution that may be suitable for the parenteral route, or mix multiple medications in the same syringe prior to administration.
Each of these preparations requires specific guidelines to be followed when preparing the medication.
Link to Learning
You can view how to withdraw medication from a vial in this video demonstration [9:28].
Parenteral Routes and Administration
Intradermal (ID) Route
ID are injections administered into the dermis, just below the epidermis. The ID injection route has the longest absorption time of all parenteral routes.
Link to Learning
You can view how to administer an intradermal medication in this video demonstration [2:48].
Subcutaneous (Subcut) Route
Medications administered via the subcut route are injected beneath the skin into the adipose tissue, just below the epidermis and dermis. Medications administered via this route have a slow, sustained rate of absorption because there are fewer blood vessels present to distribute the medication. To ensure that the medication is injected into the adipose tissue, careful consideration must be given when selecting an appropriate anatomical site for the subcut injection.
Link to Learning
You can view how to administer a subcutaneous medication in this video demonstration [3:39].
Intramuscular (IM) Route
Medications administered via the intramuscular route are injected directly into the muscle. Muscle tissue has a large blood supply; therefore, IM medications may be absorbed faster than those injected via the subcut route and also have a reasonably prolonged action. Due to their rich blood supply, IM injection sites can absorb larger volumes of solution, and in addition, muscle tissue is less sensitive than subcutaneous tissue to irritating solutions and concentrated and viscous medications.
Link to Learning
You can view how to administer an intramuscular medication in this video demonstration [2:44].
Intravenous (IV) Route
IV is a route for administering concentrated medications (diluted or undiluted) directly into the vein. Administering a medication intravenously eliminates the process of hepatic first-pass by depositing the medication directly into the blood. This results in the immediate elevation of serum drug levels and high drug concentrations in vital organs, such as the heart, brain and kidneys. To administer IV medications safely and effectively, nurses must follow all healthcare facility policies and use the appropriate resources, such as the Australian Injectables Handbook, to determine which medications can be given intravenously and any specific instructions about administration.
Safety Considerations in Parenteral Administration
Compared to oral administration, medications administered via the parenteral route have a faster onset and provide stronger effects, as they bypass the gastrointestinal tract and enter directly into systemic circulation. This direct access requires nurses to consider a range of additional factors when preparing and administering medications via this route. These include the rate of absorption, potential medication interactions, safe administration of reconstituted medication, infection control measures, and the prevention of needle stick injuries.
Rate of Absorption
Medications administered via the parenteral route bypass the gastrointestinal system, eliminating the effects of first-pass metabolism. The medications are rapidly absorbed; therefore, there is an increased risk of overdose and rapid development of life-threatening adverse reactions.
The speed of absorption may make it difficult to reverse the physiological effects of the drug.
- Subcutaneous injections have the slowest absorption rate
- Intramuscular injections have a faster absorption rate
- Intravenous medications are the most rapidly absorbed.
Potential Incompatibilities
When administering any medication, it is important to consider potential incompatibilities; this is of extra importance for those delivered intravenously. Incompatibilities for the IV route can be organised into three different categories, including physical, chemical, and therapeutic issues. Click the plus signs to find out more.
To assess medication compatibility, refer to available educational resources such as the MIMS, Australian Injectable handbook, Don’t rush to crush and the Australian Medicines Handbook.
Safe Administration of Reconstituted Medications
After a medication has been reconstituted, best practice recommends that the individual who prepared the medication also administer it. However, if this is not possible, it is essential that the syringe is clearly labelled with the following information:
- The type and amount of diluent used
- The expiration date of the diluent
- The time in which the medication must be used after being reconstituted.
This practice aligns with the National Standard for User-Applied Labelling of Injectable Medicines, Fluids and Lines. According to the guidelines, any medication that has been removed from its original packaging, is no longer recognisable and leaves the hands of the dispenser, must be clearly labelled and identifiable (ACSQHC, 2015). For further information on correct labelling, see Safety Alert: Labelling, in the Fluid Electrolyte and Acid Base chapter.
Infection Control
As the parenteral route is an invasive procedure that requires the skin integrity to be broken, the risk of introducing infection is increased. The intravenous route poses additional risks due to providing a direct entry into the systemic circulation. To reduce the risk of infection, it is therefore vital to follow infection control guidelines such as the 5 moments of hand hygiene and aseptic technique whenever preparing and administering medications.
In all parenteral administration procedures, multiple microcritical aseptic fields are utilised. The nurse needs to remain vigilant and consistently aware of key sites and key parts that require protection to maintain asepsis. The level of aseptic technique and the fields required to maintain asepsis will vary depending on the route and the access device used. For example, in intravenous access, a general aseptic field is established when accessing a central venous catheter (CVC) as opposed to the use of microcritical fields for a peripheral intravenous catheter (PIVC). For a reminder on Aseptic technique and Aseptic fields, revisit the Infection Prevention and Control chapter. For more information on Intravenous access devices, see Administering and Management of Intravenous Therapy (IV) in the Fluids, Electrolytes and Acid Base chapter.
Specifically, when handling needles, syringes and medication, the following aseptic principles should be applied:
- Prepare parenteral medications in a clean area on a clean surface
- Use single-use equipment (e.g., syringes, needles, IV sets) for one patient only
- Open packaging as it is designed, do not rip, tear or ‘pop’ open packages
- Ensure needles do not touch unsterile surfaces, such as the outside of the vial or countertop
- Disinfect vial stoppers before inserting a needle
- Ensure the tip of the needle is covered whenever it is not in use
- Avoid contaminating the length of the plunger and the Luer-lock, if using a two-piece needle and syringe (contamination of these parts has the potential to introduce contaminants into the syringe).
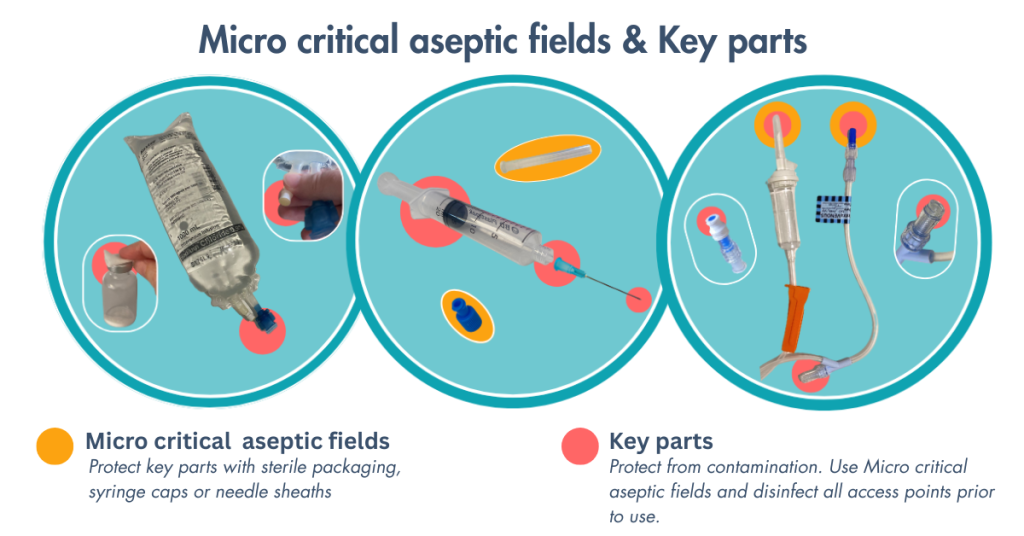
Figure 1. Use microcritical aseptic fields to protect key parts
Specific to preparing and administering IV medications, these additional steps should be followed:
- Disinfect all access points (e.g., hubs, ports, connectors) with a 70% alcohol wipe before use. Scrub for 5–15 seconds and allow to dry completely (about 5 seconds for alcohol, 20 seconds for chlorhexidine-alcohol) (NHMRC, 2019).
- Avoid disconnecting IV lines unless necessary. If disconnected, replace the administration set.
Further information can be found on infection control and safety considerations when using intravenous route in the next chapter, Fluid, Electrolyte and Acid Base.
Preventing Needle Stick Injuries
Registered nurses are at significant risk of injury when administering parenteral medications. According to the National Health and Medical Research Council (2019), 41% of sharp-related injuries occur during the use of a sharp device on a patient, 40% occur after use but before disposal, and 15% happen during or after disposal, whether appropriate or inappropriate. To reduce the risk of needlestick injury during the administration of parenteral medications, it is essential to follow established practice guidelines.
Practice Guidelines
For more information in regards to sharp safety, revisit Sharp Safety in the Foundations of Medication Safety chapter and Handling of Disposable Sharps in the Infection Prevention and Control chapter.
Key Takeaways
In this chapter, we covered:
- Nurses are responsible for ensuring safe and effective medication administration, which includes assessing the patient, understanding the medication, and evaluating outcomes post-administration.
- Parenteral medications can be administered via intradermal, subcutaneous, intramuscular and intravenous routes. The choice of route depends on the medication’s properties, therapeutic goals, and the patient’s condition.
- Parenteral medications have a relatively quick onset of therapeutic effects, and nurses must be aware of the onset, peak, and duration of all parenteral medications.
- Always consider the therapeutic effects and adverse effects when administering parenteral medications.
- Understand which medications are considered high-alert medications (APINCHS), and perform independent double checks to minimise errors.
- Report all errors, near misses, and adverse reactions to ensure knowledge is shared and to prevent further errors from occurring.
References
Australasian College for Infection Prevention and Control. (2024) Intravenous fluid (IV) preparation and management [Word doc]. https://www.acipc.org.au/wp-content/uploads/2024/10/Aseptic-Technique-IV-fluid-preparation-and-management.docx
Australian Commission on Safety and Quality in Health Care. (2015). National standard for user-applied labelling of injectable medicines, fluids and lines. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/national-standard-user-applied-labelling-injectable-medicines-fluids-and-lines
Australian Technical Advisory Group on Immunisation (2018). Australian immunisation handbook: Vaccination procedures. https://immunisationhandbook.health.gov.au/vaccination-procedures
National Health and Medical Research Council & Australian Commission on Safety and Quality in Health Care. (2019). Australian guidelines for the prevention and control of infection in healthcare. National Health and Medical Research Council. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/australian-guidelines-prevention-and-control-infection-healthcare
Chapter Attribution
This chapter has been adapted in part from:
Clinical nursing skills (2024) by Christie Bowen et al., OpenStax, is used under a CC BY licence.
Nursing advanced skills (2023) by Kimberly Ernstmeyer and Elizabeth Christman, Resources for Nursing (Open RN), Chippewa Valley Technical College, is used under a CC BY licence.
Nursing skills 2e (2023) by Open Resources for Nursing, Chippewa Valley Technical College, is used under a CC BY licence.
Media Attributions
- Protect key parts © Leisa Sanderson is licensed under a CC BY (Attribution) license

