Foundations of Medication Safety
Tracey Gooding; Leisa Sanderson; and Penelope Coogan
Learning Outcomes
In this chapter you will learn how to:
- Describe the impact of medication errors on patient outcomes and the healthcare system in Australia
- Describe the role of the Australian Commission on Safety and Quality in Health Care (ACSQHC) in promoting medication safety
- Analyse the medication use pathway to identify potential points of error and propose strategies to mitigate risks at each stage
- Apply the principles of safe medication administration in clinical practice
- Identify and explain the risks associated with high-risk medicines, including those classified under APINCHS, Schedule 8 (S8), and Schedule 4 (S4), to support safe medication administration
- Apply the “seven rights” and three checks of medication administration
- Demonstrate the use of ISBAR and closed-loop communication in medication-related handovers
- Evaluate the appropriateness of medication administration by integrating pharmacological principles, such as mechanism of action, indications, side effects, and interactions, with patient-specific factors including age, renal and hepatic function, and allergy status
- Demonstrate culturally safe strategies to involve patients and carers in medication administration, ensuring shared decision-making and respect for diverse health beliefs and literacy needs.
Introduction
Medication safety is a fundamental aspect of nursing practice, directly influencing patient outcomes and the overall quality of healthcare delivery. Nurses play a critical role in ensuring safe medication practices. Through accurate administration, educating patients, monitoring responses, and recognising and reporting medication errors, nurses can make a significant difference in promoting medication safety. This chapter will explore the relevant standards, common causes of medication errors, safety strategies, and the responsibilities of nurses in promoting medication safety within the Australian healthcare context.
The Impact of Medication Errors – Australia’s Response
In Australia, medication errors are among the most frequently reported clinical incidents in healthcare settings, with many being preventable. Research has shown that over 250,000 Australians are hospitalised each year due to medication errors, misuse, or adverse reactions, with two-thirds considered potentially preventable (Lim et al., 2021; Pharmaceutical Society of Australia, 2019).
Medication errors can differ in their nature, where they occur, and the effects they have. Some errors are detected before reaching the patient or cause little to no harm, while others may lead to serious, potentially life-threatening outcomes. Importantly, adverse drug events (ADEs) can result in patient harm, increased hospital admissions, and substantial healthcare costs (Lim et al., 2022). Patient harm from medication errors can come in many forms. The World Health Organization (WHO) have defined patient harm as:
The impairment of structure or function of the body and/or any deleterious effect arising from, or associated with, plans or actions taken during the provision of primary health care. It includes disease, injury, suffering, disability and death, and may be physical, psychological or social. (Cooper et al., 2018, p. 502)
In 2017, the World Health Organization (WHO) launched the Global Patient Safety Challenge: Medication without Harm. This initiative aimed to reduce severe and preventable medication-related harm by 50% worldwide over the next five years. Although the initial five-year period has passed, the challenge has spurred numerous initiatives within the Australian healthcare system. These efforts, led by the Australian Commission on Safety and Quality in Healthcare (ACSQHC), continue to address medication-related harm.
In 2024, the ACSQHC published an update titled Medication without Harm WHO Global Patient Safety Challenge: Australia’s Response. This document details the various goals and actions that emerged from the challenge and evaluates their current status as met, partially met, or not met. The primary focus of these goals is to reduce harm caused by errors or unsafe practices, which are linked to known weaknesses in the Australian healthcare system.
The initiative aims to enhance safety at every stage of the medication management pathway, including prescribing, dispensing, administering, monitoring, and use. Please review the diagram below, which outlines the many areas that can influence medication safety.
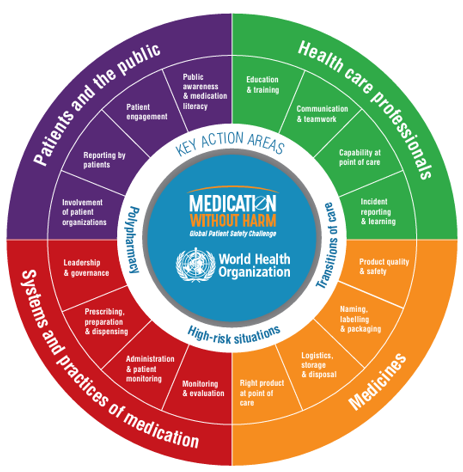
The ACSQHC’s Role in Medication Safety
The Australian Commission on Safety and Quality in Healthcare (ACSQHC) is leading Australia’s response to this challenge, with the key action areas of reducing harm from high-risk medicines, inappropriate polypharmacy and improving medication safety at transitions of care (ACSQHC, 2024b).
The ACSQHC plays a pivotal role in enhancing the safety and quality of healthcare in Australia. It develops national standards, such as the NSQHS Standards, and provides resources and tools to support their implementation. In relation to medication safety, the ACSQHC leads initiatives to reduce medication errors and adverse drug events.
The ACSQHC’s initiatives include developing national medication charts, promoting the quality use of medicines, and providing guidance on high-risk medications and providing nationally consistent medication standards (ACSQHC, 2024). In particular, the National Safety and Quality Health Service (NSQHS) Medication Safety Standard, is also supported by the Clinical Governance Standard and the Partnering with Consumers Standard (ACSQHC, 2021a).
NSQHS Medication Safety Standard
The National Safety and Quality Health Service (NSQHS) Standards, developed by the ACSQHC, provide a nationally consistent framework to ensure safe and high-quality care across Australian healthcare settings. Among these, the Medication Safety Standard specifically addresses the safe prescribing, dispensing, administering, and monitoring of medicines (ACSQHC, 2021a).
Standard 4 aims to ensure that health service organisations have systems in place to reduce the risks associated with medication use. This includes implementing policies for high-risk medicines, ensuring accurate medication histories, and promoting the safe use of medicines through staff education and patient engagement (ACSQHC, 2021a).
Link to learning
Partnering with Consumers for Medication Safety
The Partnering with Consumers Standard, developed by the ACSQHC, is all about the importance of involving patients, their families, and carers in the planning, design, delivery, and evaluation of healthcare services. This standard aims to create mutually beneficial partnerships that enhance patient care and safety (ACSQHC, 2021b).
A central component of medication safety is ensuring that patients are actively involved in their medication management. Involving patients in the decision-making process, particularly in relation to their medications, helps prevent errors, improves adherence, and encourages shared responsibility for health outcomes (ACSQHC, 2021a). Health literacy also plays a crucial role in partnering with consumers and medication safety, as patients with limited understanding of medical terminology may struggle to follow medication instructions accurately. For this reason, patients should be encouraged to ask questions about their medications and clarify any doubts. Nurses should adapt their communication strategies to meet the health literacy needs of patients, using simple language and checking for understanding through teach-back methods. For culturally safe communication, culturally appropriate tools and strategies, such as providing medication information in multiple languages or using visual aids and interpreters to overcome language barriers, can be used. Nurses have a critical role in facilitating open and clear communication with patients, ensuring they are well-informed about the medications they are prescribed, including the reason for the medication, potential side effects, correct dosages and timing, the expected duration of treatment and when to seek help if needed.
Link to learning
Communicating for Medication Safety
The Communicating for Safety Standard, developed by the ACSQHC, aims to ensure timely, purpose-driven, and effective communication and documentation that support continuous, coordinated, and safe care for patients. This standard recognises that effective communication is essential throughout a patient’s care journey, particularly during high-risk times such as medication administration and transitions of care, when critical information emerges or changes, and to ensure correct patient identification and procedure matching, in this case, when administering medication (ACSQHC, 2021a, ACSQHC, 2021b). By implementing systems and processes that support structured clinical handovers and the communication of critical information, this standard helps to reduce medication errors and enhance overall patient safety.
Link to learning
The Medication Management Pathway – It’s Everyone’s Responsibility
While nurses are often at the frontline of medication administration, all healthcare professionals share the responsibility of minimising the risk of medication errors. These errors can occur when a planned action is not completed (such as an omission) or when it is carried out incorrectly. For example, administering the wrong medication to the wrong patient, via the wrong route, at the wrong dose or at the wrong time.
Medication administration is a complex, multi-step process involving numerous individuals. This complexity introduces many potential points where errors can occur, leading to medication-related harm. The medication management pathway outlines the key stages of prescribing, dispensing, administering, and monitoring, each of which contains multiple sub-steps and decision points. The pathway provides a framework for identifying when there is potential for errors or risk of harm and responding with strategies to reduce the opportunity for error (ACSQHC, 2017b; Commonwealth of Australia Department of Health and Aged Care, 2022).
Understanding this pathway is essential for identifying where risks may arise and how they can be mitigated. Refer to the diagram below and click or press on the + sign to understand each step in the medication pathway. As you look through each stage, consider how errors could occur in each stage and reflect on the parts that you will be involved in and what you will do to prevent medication errors.
As you can see, there are many steps and people involved in the safe administration of medications. It is everyone’s responsibility to always remain vigilant when it comes to medication administration and ensure medications are prescribed, dispensed, administered and monitored safely. This is a big responsibility, but one that should be taken seriously, as it plays a critical role in preventing harm and ensuring quality care.
Foundations of Safe Medication Practice
Ensuring the safe administration of medications is a fundamental responsibility for Nurses. This responsibility is guided by well-established principles that aim to minimise the risk of medication errors and enhance patient outcomes. This section outlines key foundations of safe practice, including legal requirements for valid medication orders, essential pharmacological knowledge, and the “Seven Rights” of medication administration along with the broader considerations for the role of technology and effective communication in promoting safe medication practices.
What Makes a Valid and Legal Order
The National Inpatient Medication Chart (NIMC)
The National Inpatient Medication Chart (NIMC) is a nationally standardised set of medication charts, available in both paper and electronic formats, widely implemented across Australian healthcare settings to ensure consistent presentation and communication of medication information among healthcare professionals. This standardisation helps to minimise major risks associated with medication prescribing and administration errors. It is built around uniform processes for prescribing, dispensing, administering, and reconciling medications (ACSQHC, 2019b). By using the same chart nationwide, health professionals can become familiar with its structure and underlying safety principles regardless of where they work. All public and private health services in Australia, including day procedure centres, are required to use the NIMC (ACSQHC, 2019b). The chart is grounded in evidence-based practices and best-practice guidelines and is incorporated into undergraduate health curricula and competency frameworks for safe medication management. Its primary aim is to enhance the quality and safety of medication practices and reduce the risk of medication-related errors (ACSQHC, 2019b).
There are different versions of the NIMC depending on the setting and purpose. There are NIMCs for acute care, long-stay, paediatric, paediatric long stay, GP e-version, day surgery, Cloazapine titration chart and subcutaneous insulin chart (adult).
It is important to understand the requirements for a valid medication order. A medication order is only valid if the prescriber enters all the required items. To understand the requirements for a valid order, scroll down and read the full NIMC user guide (ACSQHC, 2019b) below.
Safe Use of Medicines Terminology
A significant contributor to medication errors is the use of unsafe abbreviations and unclear dose expressions. For this reason, Australia has established national guidelines for the terminology, abbreviations, and symbols used when prescribing and administering medications. These standards include (ACSQHC, 2024a):
• Core principles to support consistent prescribing language
• A list of recommended terms and approved abbreviations
• A compilation of high-risk abbreviations, symbols, and dose expressions known to contribute to medication errors and which should be avoided.
It is extremely important for you as a future nurse to understand these recommendations, as it is you who will be deciding whether an order is valid and correct before administration. Scroll down and read pages 3-7 of the Recommendations for safe use of medicines terminology to understand these best practice medication safety recommendations.
Considerations for Electronic Medication Management Systems (EMMS)
While many healthcare organisations now use Electronic Medication Management Systems (EMMS) to streamline the prescribing, dispensing, and administration of medicines, the same legal and safety principles that apply to paper-based systems, such as those outlined in the National Inpatient Medication Chart (NIMC) above, still apply (ACSQHC, 2019b). In fact, EMMS can improve the legibility, visibility and accuracy of medication documentation (ACSQHC, 2017a). A medication order must meet legal requirements regardless of whether it is written electronically or by hand. It is also recommended that all digital medicines information be presented in full, without abbreviations (ACSQHC, 2017). EMMS are designed to support best practice and safe prescribing by incorporating these standards into their workflows, reducing the risk of error while maintaining alignment with national medication safety guidelines.
Pharmacology Basics for Safe Practice
A foundational understanding of pharmacology is critical to the safe administration of medications. Nurses need to have knowledge of how medications work, their intended purposes, side effects, and potential drug interactions to make informed decisions in their clinical practice. Additionally, patient-specific considerations, such as underlying medical conditions and age, must be taken into account when administering medications. Click on the tabs below to get an overview of the key aspects of pharmacology that contribute to safe and effective medication practice.
The “Seven Rights” of Medication Administration
As you can see, medication safety involves a series of checks and balances designed to prevent errors. One of the foundational principles in medication safety is the concept of the “seven rights” of medication administration. These rights are a guideline for nurses to follow, ensuring that each step in the medication administration process is carried out accurately and safely. By adhering to these principles, nurses can significantly reduce the risk of medication errors and enhance patient safety.
The “Seven Rights” of medication administration serve as a foundational framework to ensure safe and effective medication practices. Click on the + in each of the rights below to learn more about each of these “7 rights” of medication administration:
Monitoring for the Effects of Medications
Effective monitoring is essential in ensuring the safe and therapeutic use of medications. Nurses play a critical role in observing patients for both therapeutic effects (the desired outcome of medication) and adverse effects (harmful or unintended reactions).
- Therapeutic Effects: Therapeutic effects refer to the beneficial outcomes of a medication, such as pain relief from an analgesic or reduced blood pressure from antihypertensive medications. Nurses must assess and document these outcomes, ensuring that the medication is providing the desired effect. For instance, when administering insulin to a diabetic patient, the nurse should monitor blood glucose levels to ensure the drug is effectively managing the patient’s condition.
- Adverse Effects: Adverse effects are unwanted side effects that can occur when taking medications. Nurses must regularly monitor patients for signs of these effects and intervene as needed. For example, opioids can cause respiratory depression, which requires careful monitoring of respiratory rate and oxygen saturation, especially during the initial phases of treatment. Nurses should also educate patients about possible side effects and encourage them to report any unusual symptoms promptly.
- Laboratory Monitoring: In addition to clinical observation, laboratory tests may be required to monitor for therapeutic and adverse effects. For example, when a patient is prescribed warfarin, the nurse must monitor the international normalised ratio (INR) regularly to ensure the drug’s anticoagulant effect is within the therapeutic range. Laboratory tests such as liver function tests, kidney function tests, and complete blood counts are crucial for detecting potential medication complications early.
The Role of Technology in Medication Safety
The integration of technology into medication management has significantly enhanced medication safety, improving efficiency, reducing errors, and providing real-time information to healthcare professionals. This section explores the role of electronic medication management systems (EMMS), the use of barcoding and scanning for medication verification, and the benefits and limitations of digital alerts, including alert fatigue.
Electronic Medication Management Systems (EMMS) are designed to enhance medication safety by streamlining medication orders, administration, and documentation. Research has found that the introduction of EMMS can reduce rates of medication errors, and in particular, prescribing errors (Gates et al., 2021). The ACSQHC supports the implementation and use of EMMS in Australian healthcare systems to improve medication safety (ACSQHC, 2019a). EMMS can not only improve legibility, standardisation and accessibility, but it can also alert clinicians to potential drug interactions, allergies, and incorrect dosages, contributing to more accurate medication administration (ACSQHC, 2019a).
Barcoding and scanning technology play a critical role in medication verification. This system involves scanning a barcode on the patient’s wristband to ensure the correct drug is administered to the right patient at the right dose and time. Barcoding medication administration (BCMA) is recognised as a valuable tool in reducing medication administration errors, but only if implemented and used correctly (Williams et al., 2025).
Effective Communication and Documentation for Medication Safety
|
Effective communication and documentation are essential components of safe medication practice. Accurate and timely communication between healthcare professionals, patients, and carers helps reduce medication errors, improves patient outcomes, and fosters a culture of safety within healthcare settings. This section explores the use of standardised communication tools such as ISBAR (Introduction, Situation, Background, Assessment, Recommendation) and closed-loop communication, the importance of clear and accurate documentation, and the role of patient and carer education in safe medication administration.
|
Using ISBAR and Closed-Loop Communication for Safe Handover and Clarification
Clear and concise communication during handover and clarification is vital in preventing medication errors. The ISBAR framework is widely used across Australian healthcare settings to structure communication during handover. ISBAR ensures that critical information is communicated in a systematic and standardised way, reducing the risk of omissions or misunderstandings (ACSQHC, 2021b).
Research indicates that using ISBAR improves communication clarity and reduces errors in patient care, including medication administration. According to Gardea-Company et al. (2023), when healthcare providers utilise ISBAR during handovers, medication-related errors decrease, as the framework helps to clarify critical medication information. ISBAR facilitates an organised exchange of information about the patient’s current status, medication orders, and any changes in treatment, which is particularly important during shift changes or transfers between departments (Yulianti et al., 2025). Click or tap through each stage of the ISBAR handover below to understand each element of this structured approach.
In addition to ISBAR, closed-loop communication is another important tool to ensure that medication orders are accurately understood. Closed loop communication is a communication technique designed to ensure clarity, prevent errors, and verify understanding, especially in high-risk tasks like medication administration (Diaz & Dawson, 2020; Doorey et al., 2020). Closed-loop communication involves the sender providing instructions, the receiver repeating those instructions back to confirm understanding, and the sender confirming that the instructions are correct. This practice is especially crucial in preventing errors in verbal orders or clarifying medication instructions. See the image below for a visual example of closed-loop communication in medication administration.
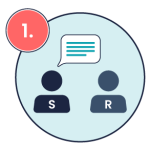 |
Sender communicates a message Example: “John, give 1mg adrenaline IV followed by a 20 mL normal saline flush.”
|
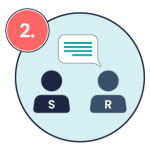 |
Receiver interprets the message, acknowledges receipt, and communicates it back to the sender Example: “OK, Mike, I am going to give 1mg adrenaline IV followed by a 20 mL normal saline flush.”
|
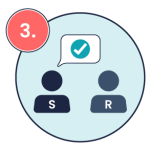 |
Sender confirms that the intended message has been received Example: “That’s correct, John.”
|
 |
Receiver reports back when the message has been acted upon Example: “Mike, 1mg adrenaline IV with a 20 mL normal saline flush has been given.”
|
Importance of Clear, Accurate, and Timely Documentation
Accurate and timely documentation is crucial for medication safety. Proper documentation ensures that all members of the healthcare team have access to up-to-date medication orders, patient responses, and any changes in treatment plans. It also provides a legal record of care provided and is essential in case of disputes or investigations into medication errors.
The ACSQHC emphasises the need for clear and legible documentation to ensure that healthcare providers can act on the correct information. According to the NSQHS Medication Safety Standard (ACSQHC, 2021a), healthcare organisations must implement systems to ensure that all medication-related documentation is complete, accurate, and readily accessible to all team members. This includes ensuring that patients’ medication charts are updated in real-time and include details on allergies, dosages, administration routes, and any changes in prescribed treatments.
According to the ACSQHC (2024a), effective documentation should meet several key standards:
Communicating Medication Incidents -The Role of Incident Reporting in Medication Safety
The ACSQHC (2021b) requires health services to implement systems for reporting and managing incidents related to the use of medicines. The aim of these systems is to learn from errors and improve medication safety. According to Khalil (2020), medication errors are the second most common type of preventable incident reported in the Australian healthcare system, but it is likely that not all medication errors or near-misses are reported. Having a blame-free culture is important in encouraging staff to report errors and near-misses, which ensures that improvements in medication safety are continuously evolving. This approach fosters an environment where employees feel safe to share their experiences without fear of retribution, ultimately leading to better practices and enhanced patient care.
Principles of Safe Medication Administration
When preparing and administering medication and assessing patients after receiving medication, always follow your healthcare facility’s policies and procedures to ensure safe and effective practice. These policies are designed to align with national safety standards and evidence-based guidelines, helping to minimise the risk of medication errors and patient harm. Review the table below for the common evidence-based principles of safe medication administration.
| Safety Considerations:
Agency policy on medication administration and medication administration record (MAR) may vary. Always receive the required training on the use of the medication system for each agency to avoid preventable errors. |
| Principle | Additional Information |
| Be vigilant when preparing medications | Minimise interruptions and distractions. Many hospitals implement “no interruption” zones (NIZ) or medication safety vests to promote uninterrupted medication preparation. |
| Check for allergies | Confirm allergies at each medication encounter. Check allergy documentation on the National Inpatient Medication Chart (NIMC) and electronic records. |
| Use three patient identifiers | Use name, date of birth, UR number, or other identifiers per hospital policy and NSQHS standards. |
| Assessment comes before medication administration | Conduct relevant pre-assessments (such as vital signs, lab values, BGL, drug levels, or other relevant assessments) prior to medication administration to ensure the patient is receiving the right medication for the right reason. Always evaluate whether the drug is still appropriate. |
| Be diligent in all medication calculations | Double-check all calculations, especially for paediatric or weight-based doses. Use calculators and seek a second check for high-risk medications. |
| Avoid reliance on memory; use checklists and memory aids | Slips in memory are caused by a lack of attention, fatigue, and distractions. Mistakes are often referred to as attentional behaviours, where a lack of training or knowledge is the cause of the error. Slips account for most errors in health care. If possible, follow a standard checklist of steps for every patient to reduce cognitive slips and lapses. |
| Communicate with your patient before and after administration | Provide information to the patient about the medication before administering it. Explain the medication’s purpose, side effects, and allow time for questions. Encourage patients to be partners in care and report any concerns. Include family members if appropriate. |
| Avoid workarounds | A workaround is a process that bypasses a procedure, policy, or problem in a system. For example, a nurse may “borrow” a medication from another patient while waiting for an order to be filled by the pharmacy. These workarounds fail to follow policy to ensure safe medication practices. |
| Ensure medication has not expired | Always check the expiry date and appearance. Do not administer if expired or altered (e.g., discolouration, sedimentation). Medications may be inactive if expired. |
| Always clarify an order or procedure that is unclear | If an order is ambiguous, incomplete, or illegible, consult the prescriber, pharmacist, or senior nurse. Nurses have a legal and professional duty to question orders. |
| Use available technology to administer medications | Electronic medication management systems and barcode scanning improve accuracy. However, be alert to issues like alert fatigue or system overrides. |
| Report all near misses, errors, and adverse reactions | Use incident reporting systems to report all near misses, errors or adverse reactions. Reporting allows for analysis and identification of potential errors, which can lead to improvements and the sharing of information for safer patient care. Reporting improves systems and is encouraged. |
| Be alert to error-prone situations and high-risk medications | High-risk medications are those that are most likely to cause significant harm, even when used as intended. The most common high-risk medications are Anti-infectives, Potassium and other electrolytes, Insulin, Narcotics/sedatives and Neuromuscular blockers, Chemotherapy and Heparin/anticoagulants. These types of medication require extra precautions, second checks, and knowledge of institutional guidelines. |
| If a patient questions or expresses concern about a medication, stop and do not administer it | If a patient questions a medication, stop, listen and explore the patient’s concerns, review the order, and, if necessary, notify senior nursing and medical staff. Patients often detect discrepancies; their input should always be taken seriously. |
Medication Safety for High-Risk Situations
Sharps Safety
 |
Nurses often need to use needles to administer medications. For that reason, sharps safety is a critical aspect of medication administration. Sharps, including needles, syringes, lancets, and other devices capable of cutting or piercing the skin, pose a significant risk of injury and infection if not handled correctly. Even a single needlestick injury can have serious health, psychological, and professional consequences for a nurse and can lead to the transfer of blood-borne pathogens.
|
Healthcare facilities have legal and professional responsibilities under workplace health and safety regulations to:
- Provide appropriate equipment and training related to sharps safety
- Minimise the risk of exposure
- Support safe reporting and follow-up procedures
- Nurses also have a duty of care to protect themselves, their patients, and colleagues by following best practice.
High-Risk Medications – APINCHS
The National Safety and Quality Health Service (NSQHS) Standard on Medication Safety mandates that health services must recognise high-risk medications (HRMs) utilised within their organisation and implement necessary measures to guarantee their safe storage, prescription, dispensing, and administration (ACSQHC, 2021a).
In the acute sector in Australia, the ‘APINCH’ acronym and classification is widely used to assist clinicians focus on a group of medicines known to be associated with high potential for medication-related harm. High-risk medicines may vary between hospitals and other healthcare settings depending on the types of medicines used and patients treated.
APINCHS is a safety acronym that helps health professionals identify medicines with a high potential for patient harm if used incorrectly. These medications are not necessarily more likely to be involved in an error. But when errors do occur, the consequences can be severe or fatal.
Click or press on each tab below to explore the high-risk medications and understand: the risks, why it is a high risk, and the associated nursing precautions you should take when dealing with these high-risk medications (ACSQHC, 2025a).
Keep in mind that the ‘APINCH’ classification does not cover all high-risk medications. Other drugs or drug classes may also carry significant risks, such as neuromuscular blockers, digoxin, antipsychotics, and oral hypoglycaemics (ACSQHC, 2025a).
There are also best practice recommendations outlined by ACSQHC that are important in improving the safety of medication administration that you should be aware of. These include being aware of the recommendations related to the use of (ACSQHC, 2025b) :
- Oral syringes – Specially designed oral syringes, which cannot be connected to IV tubing, are used for dispensing/administering oral liquid solutions.
- Infusion pumps – General infusion pumps with SMART PUMP TECHNOLOGY are in use with full functionality employed to intercept and prevent wrong dose/wrong infusion rate errors due to misprogramming the pump, miscalculation, or an inaccurately prescribed dose or infusion rate.
- Epidural pumps – Only one type of epidural infusion pump is used and is different from general infusion devices used in the organisation.
- Barcoding at point of care – If bar-coding at the point-of-care is used for medication administration, an interdisciplinary team reviews metrics from the system, including the per cent of medicines with a readable barcode, scanning compliance rates, and bypassed or acknowledged alerts, and any barriers associated with using the technology are addressed to maximise the safe use of the system.
Monitored Medicines
Scheduling is a national system used to classify medicines and chemicals based on how they are made available to the public. Each substance is placed into a specific “Schedule”, which reflects the degree of regulatory control needed to ensure public health and safety (TGA, n.d.) These Schedules are published in the The Poisons Standard (the SUSMP) and are given legal effect through state and territory legislation (TGA, n.d.). Monitored medicines include Schedue 8 (S8) medications, which are classed as “Controlled Drugs”, and some particular medicines from Schedule 4 (S4) which are also known as “Prescription Only” medicines (TGA, n.d.). When administering monitored medicines, nurses must take extra precautions due to the increased risk of harm associated with these drugs. Schedule 8 (S8) medications, such as opioids and selected benzodiazepines, and selected Schedule 4 (S4) medicines such as codeine and most benzodiazepines, have a high potential for overdose, dependence, misuse, or diversion. These medications are regulated under each state and territory law, which defines them as posing significant risks to both patients and the wider community (Queensland Health, 2025).
Nurses are responsible for adhering to strict legal, professional, and workplace protocols when handling these medicines. This includes verifying prescriptions, double-checking doses (often with another authorised health professional), accurately recording administration in designated drug registers, securely storing the medications, and reporting any discrepancies or incidents immediately. Unauthorised access or misuse of S8 or S4 drugs can have serious legal consequences. Nurses must also be vigilant in assessing the patient’s response and watching for signs of adverse effects or misuse.
It is important to note that the classification and regulatory requirements for monitored medicines vary between Australian States and Territories. For this reason, it is beyond the scope of this textbook to list all the monitored medicines. Therefore, nurses must be familiar with the specific legislation and health service policies relevant to their location and practice setting.
Special Considerations for Administering Paediatric Medications
 |
Paediatric patients are a high-risk group when it comes to medication administration. Even small miscalculations can lead to serious harm due to their size, immature organ systems, and limited ability to communicate adverse effects. Unlike adults, paediatric doses must be individually calculated based on weight, body surface area (BSA), and sometimes gestational age. |
To reduce the risk of medication errors, strict documentation and double-checking processes are required. Click each section below to expand and learn more about safe paediatric medication administration.
Bringing it all Together: A Step-by-Step Process of Safe Medication Administration
Now that we’ve explored the key safety principles of medication administration, let’s take a closer look at how these principles come together in practice.
Before administering any medication, it’s essential to follow a structured safety process. While the core principles of safe medication administration remain consistent, specific steps may vary depending on the type of medication (e.g., oral, intravenous, or controlled drugs). You will find more nuanced step-by-step processes in the following chapters. This section will just review the basic safety checks required before medication administration. Additionally, individual hospitals and healthcare services may have their own policies and procedures. You should always follow your local hospital guidelines. Use the interactive tool below to explore each stage of the medication administration process, from reviewing the order to final documentation. Click on each section to learn what to check, consider, and do at each step.
Key Takeaways
In this chapter, we covered:
- The impact of medication errors on patient outcomes and the healthcare system in Australia
- The World Health Organization (WHO) strategic framework for the global Medication Without Harm challenge
- The role of the Australian Commission on Safety and Quality in Healthcare (ACSQHC) in promoting medication safety and contributing to the WHO’s Medication Without Harm: Global Patient Safety Challenge
- The National Safety and Quality Health Services (NSQHS) Medication Safety Standard
- The Medication Management Pathway to understand the many stages and roles involved in medication administration where errors can occur at any stage in the pathway
- The importance of understanding the pharmacology basics for safe practice, including: mechanism of action, indications, side effects, allergies, drug interactions, renal and hepatic function and age
- What makes a legal and valid order, including the importance of understanding the standardised National Inpatient and Medication Chart guidelines and the recommendations for safe use of medicines terminology developed by the ACSQHC
- The “Seven Rights” of medication administration
- The evidence-based principles of safe medication administration
- The responsibilities and strategies to avoid sharps injuries
- Medications that have been identified as high-risk medications, including APINCHS and monitored medicines – S8s and selected S4s
- Best practice recommendations related to the use of high-risk medication procedures, including the use of: dedicated oral syringes, smart pump technology, only one type of epidural pump which is different from general IV pumps, and the importance of reviewing barcoding at point of care metrics
- Special considerations for administering paediatric medicines, including documenting weight, height and BSA, weight-based dose calculations, importance of double-checking all paediatric medications, gestational age at birth considerations and the importance of family-centred care in medication administration
- Monitoring the effects of medications, including therapeutic effects, adverse effects and laboratory monitoring
- Effective communication in medication safety, including the use of ISBAR, closed-loop communication and clear, accurate and timely documentation
- A step-by-step process of safe medication administration including: review of medication order, assessing the patient, 3 medication checks and ID checks, including allergy checks, administration, documentation and evaluation
- Technology and medication safety
- The importance of partnering with consumers for medication safety
- The role of incident and near-miss reporting in improving the safety of medication administration.
References
Australian Commission on Safety and Quality in Health Care. (2017a). National guidelines for on-screen display of medicines information. https://www.safetyandquality.gov.au/sites/default/files/migrated/National-guidelines-for-on-screen-display-of-medicines-information.pdf
Australian Commission on Safety and Quality in Health Care. (2017b). National safety and quality health service standards: Guide for hospitals. https://www.safetyandquality.gov.au/sites/default/files/2019-05/national-safety-and-quality-health-service-standards-guide-for-hospitals.pdf
Australian Commission on Safety and Quality in Health Care. (2019a). Electronic medication management systems: A guide to safe implementation (3rd ed.). https://www.safetyandquality.gov.au/publications-and-resources/resource-library/electronic-medication-management-systems-guide-safe-implementation-third-edition
Australian Commission on Safety and Quality in Health Care. (2019b), National inpatient medication chart user guide. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/national-inpatient-medication-chart-nimc-user-guide
Australian Commission on Safety and Quality in Health Care. (2021a). Medication safety standard. https://www.safetyandquality.gov.au/standards/nsqhs-standards/medication-safety-standard
Australian Commission on Safety and Quality in Health Care. (2021b). National safety and quality health service standards (2nd ed.). https://www.safetyandquality.gov.au/publications-and-resources/resource-library/national-safety-and-quality-health-service-standards-second-edition
Australian Commission on Safety and Quality in Health Care. (2024a). Recommendations for safe use of medicines terminology. https://www.safetyandquality.gov.au/our-work/medicines-safety-and-quality/safer-naming-and-labelling-medicines/recommendations-safe-use-medicines-terminology
Australian Commission on Safety and Quality in Health Care. (2024b). Status report: Medication Without Harm – WHO Global Patient Safety Challenge: Australia’s response. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/status-report-medication-without-harm-who-global-patient-safety-challenge-australias-response
Australian Commission on Safety and Quality in Health Care. (2025a, June). APINCHS classification of high risk medicines. https://www.safetyandquality.gov.au/our-work/medicines-safety-and-quality/high-risk-medicines/apinchs-classification-high-risk-medicines
Australian Commission on Safety and Quality in Health Care. (2025b, June). High-risk medicines resources. https://www.safetyandquality.gov.au/our-work/medication-safety-and-quality/high-risk-medicines/high-risk-medicines-resources
Commonwealth of Australia Department of Health and Aged Care. (2022). Achieving continuity in medication management: Guiding principles. https://www.health.gov.au/sites/default/files/2022-11/guiding-principles-to-achieve-continuity-in-medication-management.pdf
Cooper, J., Williams, H., Hibbert, P., Edwards, A., Butt, A., Wood, F., Parry, G., Smith, P., Sheikh, A., Donaldson, L., & Carson-Stevens, A. (2018). Classification of patient-safety incidents in primary care. Bulletin of the World Health Organization, 96(7), 498–505. https://doi.org/10.2471/BLT.17.199802
Diaz, M. C. G., & Dawson, K. (2020). Impact of simulation-based closed-loop communication training on medical errors in a pediatric emergency department. American Journal of Medical Quality, 35(6), 474–478. https://doi.org/10.1177/1062860620912480
Doorey, A. J., Turi, Z. G., Lazzara, E. H., Mendoza, E. G., Garratt, K. N., & Weintraub, W. S. (2020). Safety gaps in medical team communication: Results of quality improvement efforts in a cardiac catheterization laboratory. Catheterization and Cardiovascular Interventions, 95(1), 136–144. https://doi.org/10.1002/ccd.28298
Gadea-Company, P., Casal Angulo, C., & Hurtado Navarro, C. (2023). Impact of the implementation of Identification-Situation-Background-Assessment-Recommendation. PloS One, 18(6), Article e0286565-. https://doi.org/10.1371/journal.pone.0286565
Gates, P. J., Hardie, R.-A., Raban, M. Z., Li, L., & Westbrook, J. I. (2021). How effective are electronic medication systems in reducing medication error rates and associated harm among hospital inpatients? A systematic review and meta-analysis. Journal of the American Medical Informatics Association : JAMIA, 28(1), 167–176. https://doi.org/10.1093/jamia/ocaa230
Lim, R., Ellett, L. M. K., Semple, S., & Roughead, E. E. (2022). The extent of medication-related hospital admissions in Australia: A review from 1988 to 2021. Drug Safety, 45(3), 249–257. https://doi.org/10.1007/s40264-021-01144-1
Pharmaceutical Society of Australia. (2019). Medicine safety: Take care – Annual report. https://www.psa.org.au/wp-content/uploads/2019/01/PSA-Medicine-Safety-Report.pdf
Queensland Health. (2025). Prescribing monitored medicines. State of Queensland. https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/medicines/monitored-medicines/prescribing
Therapeutic Goods Administration. (n.d.) Scheduling basics of medicines and chemicals in Australia. Commonwealth of Australia. https://www.tga.gov.au/how-we-regulate/ingredients-and-scheduling-medicines-and-chemicals/scheduling-basics-medicines-and-chemicals-australia
Williams, R., Kantilal, K., Man, K. K. C., Blandford, A., & Jani, Y. (2025). Barcode medication administration system use and safety implications: A data-driven longitudinal study supported by clinical observation. BMJ Health & Care Informatics, 32(1), Article e101214. https://doi.org/10.1136/bmjhci-2024-101214
World Health Organization. (2017). Strategic framework of the global patient safety challenge – Medication without harm. https://www.who.int/initiatives/medication-without-harm
Yulianti, L., Rusca Putra, K., Supriati, L., Wiji Utami, Y., Djanatunisah, A. & Yuniarsih, W. (2025). Introduction Situation Background Assessment Recommendation (ISBAR) checklist to improve nurses’ handover quality. Babali Nursing Research, 6(2). https://doi.org/10.37363/bnr.2025.62476
Chapter Attribution
This chapter has been adapted in parts from:
Clinical Procedures for Safer Patient Care (2015) by G. R. Doyle and J. A. McCutcheon is used under a CC BY licence.
Media Attributions
- Strategic framework of the global patient safety challenge – Medication without harm © World Health Organisation is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Different types of yellow sharps bins © Tracey Gooding is licensed under a CC BY-NC (Attribution NonCommercial) license

