4.3.1 Compounding – Rheology
The Fundamentals of Rheology
Learning Outcomes
Be able to:
- Explain rheology and different types of flow behaviour of pharmaceutical liquids
- Describe Newton’s law of flow and its application
- Define different terms and concepts of Non Newtonian Fluids
- Identify Newtonian and Non Newtonian fluids from a rheogram
- Apply rheology in pharmaceutical sciences.
Viscosity
 |
 |
The science and physics of how liquids flow and solids are deformed form the basis of what is known as rheology.
But what is the importance of rheology in the pharmaceutical industry? It is important to understand the behaviour of fluids and other materials in pharmaceutical manufacturing and formulation. The viscosity of a suspension changes the rate of sedimentation, if the viscosity is too low the drug particles may settle quickly making the dosage inconsistent, a higher viscosity ensures a more uniform suspension.
The viscosity is also used as a measure of resistance to flow. In practical terms this reflects on pouring a dose from a bottle or drive a dose through a syringe. Therefore, controlling viscosity is crucial to ensure effective dosage and administration.
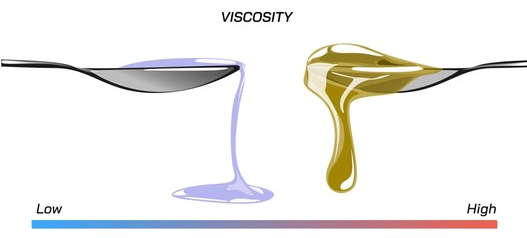
Fuids flow at different speeds, making their viscosity one of their most important properties. Viscosity is the amount of internal friction within a fluid that determines its consistency.
” The Viscosity of a fluid may be described simply as its resistance to flow or movement”.
For example – Water, which is easier to stir than syrup is said to have a LOWER viscosity.
Newtonian Fluids
What makes a fluid either “Newtonian” or “non-Newtonian”? In 1687 Sir Isaac Newton described Newtonian Fluids in Newtons Law of Viscosity.
He was the first to realise that the rate of flow (γ) is directly related to the applied stress (σ): the constant of proportionality is the coefficient of dynamic viscosity (η), more usually referred to as the viscosity.
Simple fluids which obey this relationship are referred to as Newtonian fluids and those which do not are known as non-Newtonian fluids.
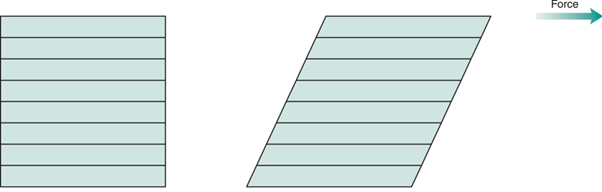
The phenomenon of viscosity is best understood by a consideration of a hypothetical cube of fluid made up of infinitely thin layers (laminae) which are able to slide over one another like playing cards in a pack or deck When a force is applied to the uppermost layer, it is assumed that each subsequent layer will move at progressively decreasing velocity and that the bottom layer will be stationary
A velocity gradient will therefore exist, and this can be calculated by dividing the velocity of the upper layer by the height of the cube. The resultant gradient, which is effectively the rate of flow but is usually referred to as the rate of shear or shear rate, γ. The applied stress, known as the shear stress, σ, is derived by dividing the applied force by the area of the upper layer.
As Newton’s law can be expressed as σ = η γ then η= σ/γ
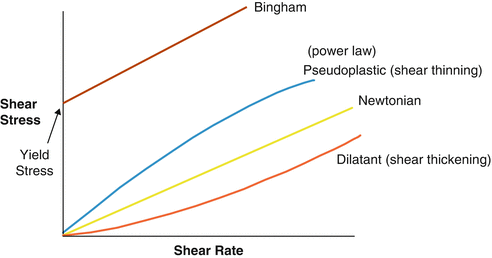
In summary, Newtonian fluids have a constant viscosity or flow, and that their flow behaviour only changes when there is a change in temperature or pressure. Stress does NOT affect this type of fluid.
With Newtonian fluids, the relationship between sheer stress and sheer rate is linear.
Newtonian fluids include water, air, alcohol, and glycerol.
Let’s look at WATER: Temperature affects its viscosity. At 0˚C it turns into a solid, and at 100˚C it is a gas, but between those temperatures is behaves like a normal fluid and has a constant viscosity.
The viscosity of water is NOT affected by applied stress.
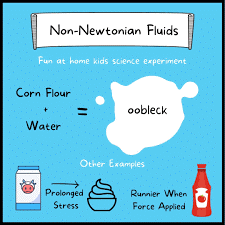
Non-Newtonian fluids DO NOT follow Newton’s law of viscosity. In non-Newtonian fluids, the viscosity changes when under force/stress to either more liquid or more solid. In simple terms, if you apply a force to such fluids (say you hit, shake or jump on them), the sudden application of stress can cause them to get thicker and act like a solid, or in some cases it results in the opposite behaviour, and they may get runnier than they were before. Remove the stress (let them sit still or only move them slowly) and they will return to their earlier state.
Non-Newtonian fluids include some slat solutions, polymers and then more recognisable ketchup, cream, custard, blood and oobleck!
🎞️ Let’s watch a video: ⏰ 5 minutes
This video is amazing!
Oobleck is easy to make and is commonly used to display the properties of non-Newtonian fluids. Oobleck is made from cornstarch and water, and the starch components are suspended in water which causes its unusual behaviour. When force is applied, the liquid turns into a solid.
For example, you could easily walk/run over a large container of oobleck because of the forces applied by your weight causes the oobleck directly under your feet to become solid. After the force is removed, the oobleck will return to its original state of liquid.
🎞️ Let’s watch a video: ⏰ 6 minutes
Sheer Stress and Sheer Rates
Sheer stress is the resistance of the movement of the surfaces, or the force applied parallel to a surface. In Rheological terms, it’s the force required to make a material flow
Shear rate is the rate at which adjacent layers of a fluid can move past each other. with respect to each other indicating the speed of flow. Think of a liquid pharmaceutical formulation such as a syrup or suspension. When you pour the liquid from a bottle the sheer rate of the bottle’s mouth is high. As the liquid flows down the shear rate decreases.
Understanding shear rate is used in the design of packaging and dosing systems to ensure that the formulation can flow easily during administration without causing issues like clogging.
For example in oral suspensions shaking the bottle is the applied sheer stress which helps to disperse the drug particles throughout the liquid.
The rate at which the particles move determines how well they stay suspended. If the sheer rate is too low this will lead to settling. If the sheer rate is too high, it may affect the stability of the formulation
Newtonian fluids have a constant viscosity regardless of the applied stress or sheer rate. The relationship between the sheer stress and sheer rate is linear.
Saline or glucose Solutions are considered Newtonian fluids, as their viscosity remains constant. This allows for predictable and constant flow, which is crucial for accurate dosing and administration, such as with IV fluid.
Non-Newtonian fluids exhibit a varying viscosity depending on the applied stress or sheer rate, the relationship between sheer stress and sheer rate is nonlinear.
There are a number of different types of non-Newtonian fluids:
Sheer thinning pseudoplastic or plastic (Bingham Plastic) viscosity decreases with increasing sheer rate
Sheer thickening dilatant viscosity increases with increasing sheer rate.
🎞️ Let’s watch a video on non-Newtonian fluids: ⏰ 12 minutes
Rheology in Suspensions
Ideal suspending agents should exhibit high viscosity at low shear rate (slow down the rate of sedimentation or remain suspended permanently on storage) and should have a low viscosity at high shear rate (i.e., it should be free flowing during shaking and pouring)
Flocculated system shows pseudoplastic or plastic behaviour under shear, also shows thixotropic behaviour.
Deflocculated systems exhibit Newtonian flow; however, may exhibits dilatancy if high conc of dispersed phase is used.
A suspending agent, which shows thixotropic and pseudoplastic behaviour, is useful because it forms a gel on standing (storage) and becomes fluid on shaking.
Rheology in Emulsions
Most emulsions show both plastic and pseudoplastic flow rather than simple Newtonian flow. If the concentration of dispersed phase is low (< 0.05%) → the system is Newtonian
The pourability, spread ability and syringe ability of an emulsion can be determined by rheological properties.
The high viscosity of w/o emulsions creates problem with IM administration. Conversion to a multiple emulsion (w/o/w) leads to decrease in viscosity and improve ease of injection.
When concentration of dispersed phase is increased → the system becomes pseudoplastic (more viscous)
At high concentration of dispersed phase → the system becomes plastic
The higher the concentration of emulsifying agents, higher the viscosity. The higher the suspending agents, higher the viscosity
Pharmaceutical Applications
Poloxamers (Pluronics) are excipients used as surfactants, solubilising agents and emulsifying agents. They form a clear gel in water (at low conc and low temp, Newtonian); however, at high conc and temp some poloxamers exhibit “sol-gel” form (non-Newtonian).
Polymer solutions as ophthalmic wetting solutions for contact lenses
Tear replacement solutions (dry eye syndrome): 0.1-0.2% sodium hyaluronate (Hylo-Fresh and Hylo-Forte). Natural tears show shear thinning properties therefore, a suitable tear substitute should have similar properties. Viscous solution shows longer contact time compared to that of less viscous solutions.
Rheology in Practice
We can apply the principles of Rheology in the following ways in pharmacy and medicine:
Liquids
- Mixing for both Newtonian (solutions) and Non-Newtonian fluids (suspensions, emulsions). Settling and sedimentation of solid particles in suspensions are dependent on the viscosity of fluids (based on Stoke’s law); suspension should be pourable.
- The rate of creaming and coalescence in emulsions
- Viscosity of ophthalmic solutions changes with increased contact time
- Passage through orifices (pouring in a small bottle, tubes, and passage through hypodermic needles)
- Fluid transfer (infusion of blood products)
- Physical stability (phase separation)
Semisolids
- Spreading and adherence of semisolids products on the applied areas (skin) and removal from jars (collapsible tubes)
- Content uniformity (shear thickening system)
- Drug incorporation into semisolid dosage forms (ointments, creams etc); drug release from the bases (creams, ointments, gels, etc)
Pharmaceutical processing
- Capacity of equipment (filling of 1 ml viscous solution in a vial)
- Processing efficiency
Solids
- Flow of powders from hoppers
- Flow of powder during tableting and filling of capsule
- Packageability of powders
Blood
- Rheological property (i.e., the viscosity) of blood in normal and diseased states is different
- Anticoagulants (warfarin, heparin) affects the viscosity of blood (i.e., reduce viscosity) -Blood Thinner
- Aspirin causes to reduce the viscosity of blood – Blood Thinner
- Thrombolytic agents (plasminogen activator, streptokinase) and calcium channel blockers (verapamil, diltiazem, nifedipine) reduce the viscosity of blood- Blood Thinner
Pastes
Pastes are a semi-solid topical preparation where the drug particles are dispersed through a hydrophobic base such as white soft paraffin, liquid paraffin or emulsifying waxes. Preservatives are not required in these types of preparations as there is no hydrophilic component. Pastes are stiffer and thicker than ointments as they contain large amounts of powdered solids (usually 20-50%). This high percentage of powder means that pastes are also less greasy than ointments, and their “stiffness” means that they allow the application of active ingredients to a localised area (as they do not spread well). Pastes are useful when a thick relatively impermeable film is required, for example, the treatment and prevention of nappy rash, and the high powder content also means that they are useful for absorbing exudate from skin lesions that are oozing or crusting.
📚 Read/Explore
- Aulton’s Pharmaceutics (Sixth Edition) 2022 Chapter 6
With Thanks to Dr Martina Mylrea and Dr Robi Islam.
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING
This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.
Poloxamers (Pluronics) are excipients used as surfactants, solubilising agents and emulsifying agents. They form a clear gel in water (at low conc and low temp, Newtonian); however, at high conc and temp some poloxamers exhibit “sol-gel” form (non-Newtonian).
Polymer solutions as ophthalmic wetting solutions for contact lenses
