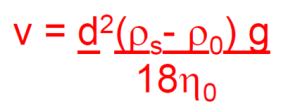4.3 Compounding – Emulsions and Suspensions
Learning Outcomes
Be able to:
- Define pharmaceutical emulsions and emulsifying agents, theory of emulsions.
- Describe the pharmaceutical application of emulsions and suspensions
- Identify the types of emulsions.
- List the classifications of emulsions instability
- Describe the HLB value and its application in emulsions
- Summarise the steps in the preparation of emulsions and prepare
- Describe the stability of emulsions.
Learning Outcomes
Be able to:
- Describe pharmaceutical suspensions and their application
- List the desirable quality of suspension
- Explain the interfacial properties of suspensions
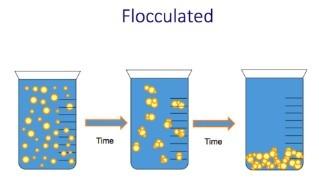
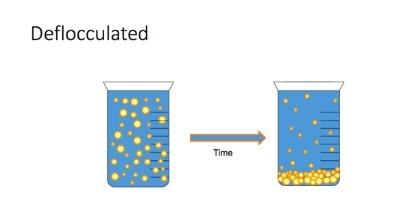
- Summarise the factors that affect the stability of suspensions (flocculation and deflocculation)
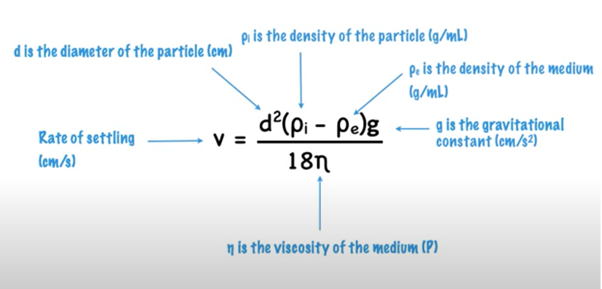
- Describe the Theory of settling and sedimentation, Stokes Law.
- Explain the sedimentation parameters, sedimentation volume and degree of flocculation
- List the formulation and preparation of physically stable suspensions.
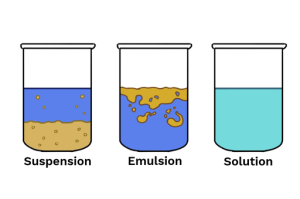
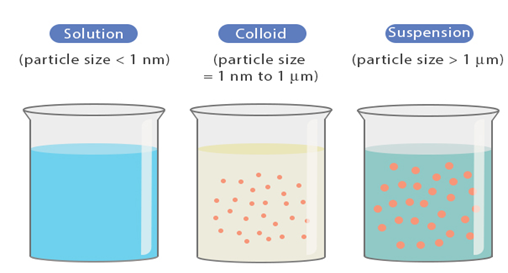
What is a suspension?
A suspension is a heterogeneous system consisting of two phases, a coarse dispersion (dispersed phase) which are finely divided insoluble particles (>0.5mm) are dispersed (suspended) uniformly in a liquid (the continuous phase OR dispersing medium)
- The concentration of dispersed phase/particles range from 0.5-30%
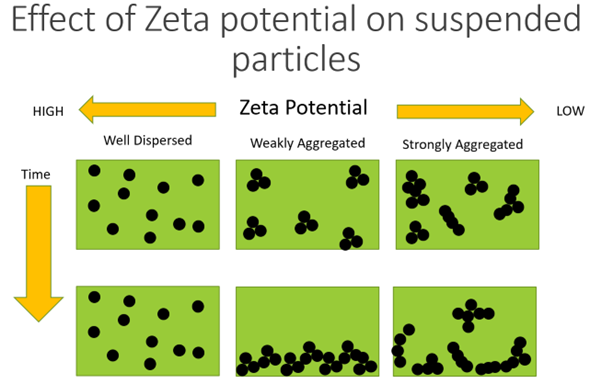
- As with emulsions, the dispersed systems are thermodynamically unstable as the small particles are highly energetic and tend to aggregate – floccule, or cake
- To get a stable product, the system needs to reduce surface free energy – reduce surface tension
Why do we need suspensions?
Suspensions are often used as a dosage form when the drug is insoluble in water and when use of solubilising agents are not possible.
- Certain drugs are chemically unstable in solution but are stable when suspended e.g., penicillin
- Dosage forms for people with swallowing difficulties such as children. e.g., Paracetamol or Ibuprofen Suspensions
- Products that are insoluble, such as minerals or inorganic salts. e.g., Calamine Lotion, salts in heartburn medication
- Flexibility in administration of a range of doses – especially when dosing is based on weight. e.g., antibiotics/ parenteral
- Used with medications that may be otherwise unpalatable unless in suspension and flavoured. e.g., antibiotics
Pharmaceutical applications of suspensions
Pharmaceutical, suspensions are used in a wide range of applications, although oral dosing is the most common.
- Oral administration: antibiotics (amoxicillin, Cephalexin oral suspension), antiacid suspensions, paracetamol & ibuprofen suspensions
- Topical administration: Calamine lotion
- Ocular administration: Suspensions for eyes e.g., Flarex eye drops
- Parenteral administration: antibiotics, vaccines
- Suspension for inhalation therapy: aerosols
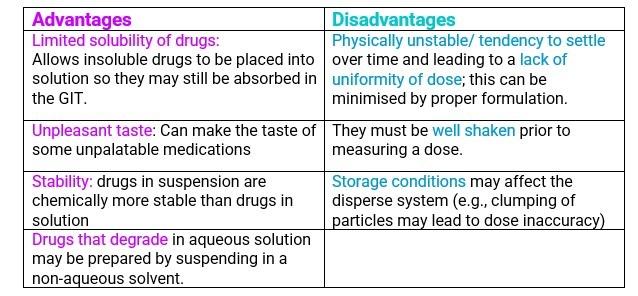
There are advantages and disadvantages of suspensions
📚 Read/Explore
- Question: Why do we need to “shake well before use”, what happens when we sell or dispense a suspension to a patient and why do we attach the ancillary label?”
- Answer: Shaking will uniformly distribute the API particles throughout the bottle. If the API particles were not uniformly distributed, then the patient would get the wrong dose. The dose will be either too low if the top of the product is used, or too high particles will be of a greater concentration of towards the bottom of the bottle if not shaken properly.
- Question: What happens to a suspension after it had been stored for a period of time, like on the shelf in the pharmacy?
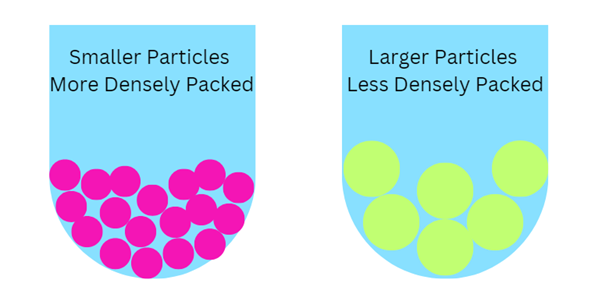
- When particles settle, they form sediment. When a suspension contains small particles that settle slowly the sediment that forms will be small and closely packed so it will be fairly dense.
-
- Dense, well packed sediment will be hard to redisperse on shaking. The consumer will have to shake the bottle very hard and may not even be able to fully redisperse the particles. To have a sediment that is easy to redisperse, we want it not well packed with lots of space between the particles. Larger particles will settle faster but won’t be as densely packed, and the sediment will be and the easier to redisperse.
- BUT REMEMBER – If the particles are too large, they may be easier to redisperse, but they will be gritty.
- How do I to get smaller particles to settle faster, but be easier to redisperse?
- By adding another excipient. Usual excipients of a suspension are the same as for a solution:
- API
- Flavourant
- Colourant
- Preservative
- Vehicle.
- By adding another excipient. Usual excipients of a suspension are the same as for a solution:
Suspending Agent
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING
This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.