4.1.1 Introduction to Heart Failure
Introduction to Heart Failure
 |
 |
 |
The National Heart Foundation defines heart failure as “a complex clinical syndrome with typical signs and symptoms that generally occur on exertion, but can also occur at rest (particularly when lying flat)”. Heart failure is secondary to an abnormality of cardiac structure or function that impairs the ability of the heart to either:
1. Fill with blood at normal pressure, which is known as heart failure with preserved ejection fraction (HFpEF)
OR
2. Eject blood sufficient to fulfil the needs of the metabolising organ, which is known as heart failure with reduced ejection fraction (HFrEF)
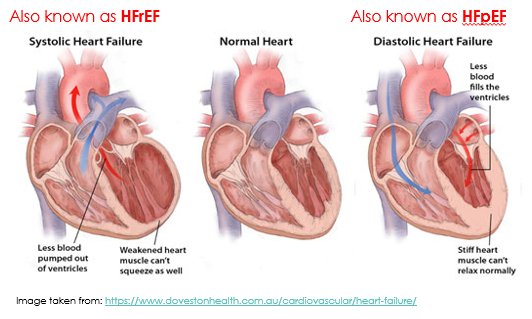 Heart failure with reduced ejection fraction (HFrEF) is also known as systolic heart failure because there is a weakened ability of the heart to contract during systole. When you look at the heart on the left hand side of the figure below, you can see weakened heart muscle of the left ventricle, meaning the heart can’t push blood out with sufficient force. There is still capacity for plenty of blood to fill the ventricle, but the heart muscle isn’t strong enough to pump it out.
Heart failure with reduced ejection fraction (HFrEF) is also known as systolic heart failure because there is a weakened ability of the heart to contract during systole. When you look at the heart on the left hand side of the figure below, you can see weakened heart muscle of the left ventricle, meaning the heart can’t push blood out with sufficient force. There is still capacity for plenty of blood to fill the ventricle, but the heart muscle isn’t strong enough to pump it out.
Heart failure with preserved ejection fraction (HFpEF) is also known as diastolic heart failure because there is impaired filling of the ventricle during diastole. When you look at the heart of the right hand side of the figure below, you can see left ventricular hypertrophy (a thicker muscle surrounding the left ventricle) that has reduced the size of the ventricle meaning less blood can fill the ventricle. On its own, HFpEF or diastolic heart failure means there is nothing wrong with the contractile ability of the heart. Instead, the problem is during the relaxation phase. HFpEF (diastolic heart failure) may occur with or without concurrent impairment of systolic function (HFrEF).
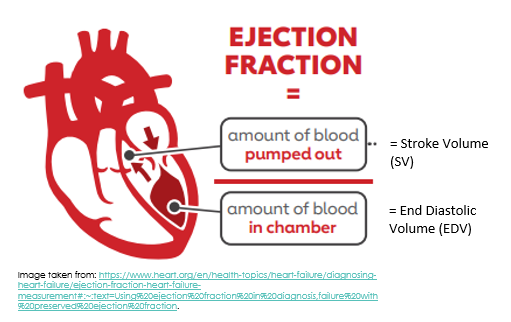
The Ejection Fraction (%)
The ejection fraction is a measure of how much blood the left ventricle (LV) pumps out with each contraction, expressed as a percentage. You can also consider it to be a ratio, whereby the stroke volume (or the amount of blood pumped out of the left ventricle) is divided by the end diastolic volume (or the amount of blood that was present in the left ventricle just before the ventricles contracted).
The ejection fraction is expressed as a percentage, with a normal ejection fraction generally considered to be between 50 and 70%. For example, if the left ventricular end diastolic volume (EDV) is 120ml and the stroke volume is 80ml, then the ejection fraction is 80/120 x 100 = 66.7%. Left Ventricular Ejection Fraction (LV EF) of 66.7% is normal.
📺 Watch the vodcast on the introduction to heart failure. (8:57 minutes)
Epidemiology
Heart failure affects at least 38 million people worldwide, including approximately 480,000 adults in Australia (which was about 2.1% of the adult Australian population in 2014). The prevalence of heart failure increases with age. Heart failure is also 1.7 times more prevalent in Indigenous populations than in non-Indigenous populations. This is primarily because of very high rates of rheumatic heart disease in Indigenous Australian populations.
- Rheumatic heart disease is damage to one or more heart valves that has been caused by an episode (or recurrent episodes) of acute rheumatic fever which is brought on by a throat or skin infection with group A streptococcus bacteria. In rheumatic heart disease, the heart becomes inflamed, and the heart valves stretched and/or scarred. If left untreated, that can readily result in heart failure.
- Indigenous Australians also have high rates of cardiovascular risk factors, and we know that acute coronary syndromes can cause heart failure.
Heart failure is also a significant burden on Australian healthcare resources. In 2015-16 there were approximately 173,000 hospitalisations due to heart failure, which was about 1.6% of all hospitalisations during that period. Survival rates for chronic heart failure range from 80-90% at 1 year. On the flip side, you can consider that this means mortality rates for chronic heart failure are 10-20% at 1 year. After 5 years, the mortality rate is up to 50%, which has been compared to that of some non-haematological malignancies.
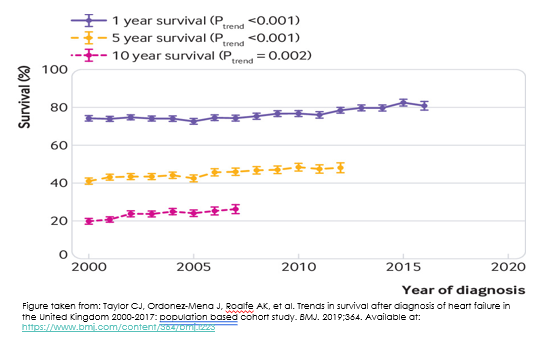
The figure below comes from a report of short- and long-term survival rates for heart failure patients from the United Kingdom. You can see all 3 lines are trending upwards. Although the data stops at 2017, we can probably safely extrapolate that someone diagnosed with heart failure today will have a slightly better 1-, 5- and 10 year survival than someone who was first diagnosed with heart failure in 2000. We can attribute such improvements to evidence-based pharmacological treatment regimens.
Aetiology of Heart Failure
Common causes of heart failure include:
- Coronary artery disease
- Including previous myocardial infarction
- Hypertension
- High blood pressure increases ventricular afterload because high pressures in the arterial circulation make it much more difficult for the heart to pump more blood out into these arteries against their high pressures
- So, optimising the treatment of any concomitant hypertension will be an essential adjunct to a standard heart failure drug treatment regimen
- Arrhythmias, especially tachyarrhythmias
- Arrhythmias assault the heart muscle in 2 ways (first by reducing diastolic ventricular filling time which will reduce stroke volume, and second by increasing myocardial oxygen demand)
- Valvular heart disease
Some less common causes of heart failure include:
- Congenital heart diseases
- Metabolic disorders (e.g., thyroid disease, diabetes, phaeochromocytoma)
- Infection and associated inflammation
- Certain nutritional deficiencies
- Radiation therapy, and
- Chemical toxins (e.g., excess alcohol intake, illicit drugs, and cardiotoxic cancer chemotherapy protocols)
📺 Watch the vodcast on the aetiology of heart failure. (8:57 minutes)
Pathophysiology of Heart Failure
Both HFrEF and HFpEF are associated with reduced cardiac output, which has many negative consequences. However, the pathophysiology is different depending on whether the diagnosis is HFrEF or HFpEF. Pathophysiology of HFrEF is well understood, however unfortunately the pathophysiology of HFpEF is not and treatments that are effective for HFrEF aren’t as effective in HFpEF.
We’ll focus on the pathophysiology of HFrEF for the purposes of this subject.
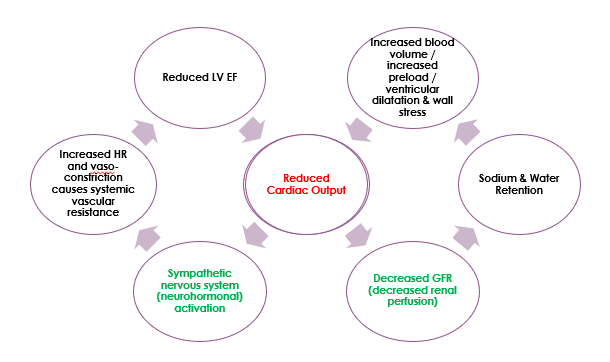
In HFrEF, reduced end-organ perfusion causes activation of compensatory mechanisms which are beneficial in the short-term but detrimental in the long-term.
Remember cardiac output = heart rate (HR) x stroke volume (SV).
Compensatory mechanisms include:
- Sympathetic Nervous System (SNS) activation. Increases heart rate to increase cardiac output. To increase cardiac output in the presence of a low SV, the body tries to increase HR. Mechanism: the body does this by activating the sympathetic nervous system, releasing adrenaline and noradrenaline, which increases HR but also cause vasoconstriction, which increases systemic vascular resistance making it more difficult for the heart to push blood out against high systemic pressures. This impairs LV EF even more. Hence, it is important to control hypertension in patients with heart failure.
- Renin-Angiotensin-Aldosterone System (RAAS) activation. Increases end diastolic volume (EDV, also known as preload). Increasing EDV increases stroke volume (SV). Remember EF = SV / EDV, therefore SV = EF x EDV. Mechanism: reduced cardiac output causes decreased perfusion of the kidneys. Because of their lack of perfusion, the kidneys activate the renin-angiotensin-aldosterone system. Aldosterone causes sodium and water retention by the kidney which increases circulating blood volume. Increasing preload in this manner will stretch the myocardium and increase SV, but only up to a point, beyond which the myocardium will be stretched too far, and cardiac output will again be worsened.
- Cardiac Remodeling, including myocardial hypertrophy and LV dilatation, attempts to increase contractility. Increased preload and the stress this puts on the ventricle walls leads to increased ventricular mass and increased contractility (initially). However, this is problematic when myocardial fibrosis stiffens the ventricle, reducing its ability to relax and predisposing to diastolic heart failure.
This pathophysiology is summarised in the diagram below. Start in the middle of the diagram, where ‘reduced cardiac output‘ is written in red. And remember that in HFrEF, cardiac output is reduced because stroke volume is reduced.
In HFrEF, compensatory mechanisms are targets for pharmacological intervention. Which drugs target compensatory mechanisms in HFrEF?
- Beta blockers are useful in heart failure to reduce sympathetic nervous system (SNS) activation. However, we must use them with caution. There is a fine balance between maintaining a heart rate that is fast enough to support sufficient cardiac output but not so fast that it increases systemic resistance too much.
- ACE inhibitors and angiotensin receptor antagonists are used to prevent sodium and water retention that is caused by activation of the renin angiotensin aldosterone system.
- And diuretics are used to reduce circulating blood volume and preload.
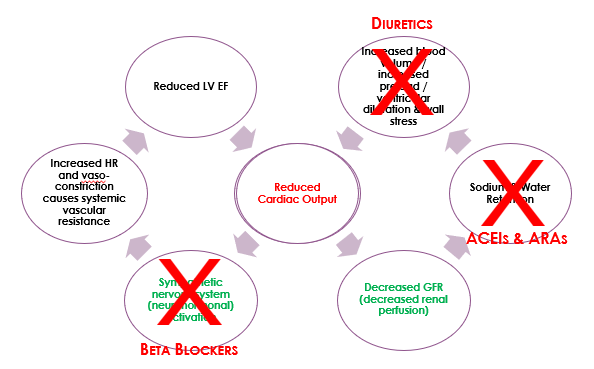
- Sacubitril is a drug that works to increase circulating concentrations of natriuretic peptides. Natriuretic peptides include atrial natriuretic peptide (ANP), brain (B-type) natriuretic peptide (BNP), and c-type natriuretic peptide (CNP) that work to cause vasodilation and salt/water excretion. They naturally counterbalance compensatory mechanisms 1 and 2 (SNS-induced vasoconstriction and RAAS-induced increased blood volume). This is important because it is another target for pharmacological intervention, but we will talk more about them later.
Signs and Symptoms of Heart Failure
The clinical syndrome of heart failure has typical symptoms; however, they are often not specific. Several other diagnoses can manifest similarly. For example, one of the most common symptoms of heart failure is dyspnoea, which can also be associated with respiratory conditions (asthma/COPD) or a pulmonary embolism or an acute coronary syndrome. Other typical symptoms of heart failure include orthopnoea which is dyspnoea but only when the patient is lying down. Asking the patient how many pillows they use to sleep at night can help to gauge whether orthopnoea is an issue. Patients can find that propping themselves up with pillows can help them to get some relief from pulmonary congestion which some patients say makes them feel like they are drowning.
The clinical presentation of heart failure can vary depending on whether the heart failure is left ventricular failure, right ventricular failure, or biventricular failure. Usually, left ventricular failure happens first. Due to circulatory dynamics, left ventricular failure often leads to right ventricular failure, and so most patients suffer from signs and symptoms of biventricular heart failure.
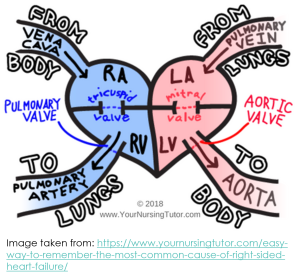
Left vs Right Sided Heart Failure
When we think about how symptoms differ between left and right sided heart failure, it is important to remember that the left side of the heart receives oxygenated blood from the pulmonary circulation and delivers it to the arteries of the body, whereas the right side of the heart receives deoxygenated blood from the body and pumps it to the lungs so it can be re-oxygenated.
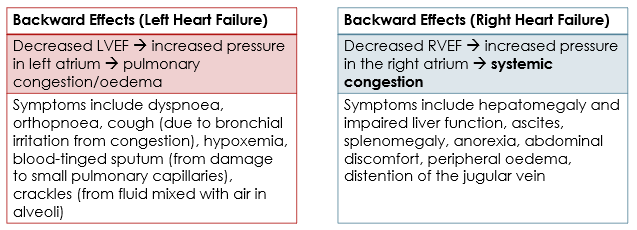
Signs and symptoms of heart failure can be classified as either ‘forward effects’ or ‘backward effects’.
- Forward effects are the direct result of reduced cardiac output.
- Backward effects are the result of congestion of blood behind the ventricle.
- LV failure ⇒ reduced tissue perfusion, tiredness, fatigue, confusion, activation of RAAS, increased heart rate, etc.
- RV failure ⇒ reduced output of deoxygenated blood to the pulmonary artery ⇒ reduced delivery of oxygenated blood to the left side of the heart ⇒ reduced tissue perfusion, tiredness, fatigue, confusion, activation of RAAS, increased heart rate, etc.
Backward effects are different for left- and right-ventricular failure.
 📺 Watch the vodcast on clinical presentation of heart failure. (8:57 minutes)
📺 Watch the vodcast on clinical presentation of heart failure. (8:57 minutes)
Download the lecture notes here:
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING
This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.