4.2.4 Renal Replacement Therapies
Learning Outcomes
Be able to:
- Define the key principles of peritoneal dialysis
- Describe the key principles of haemodialysis
- Explain if/when renal transplant may be indicated.
Renal Replacement Therapies
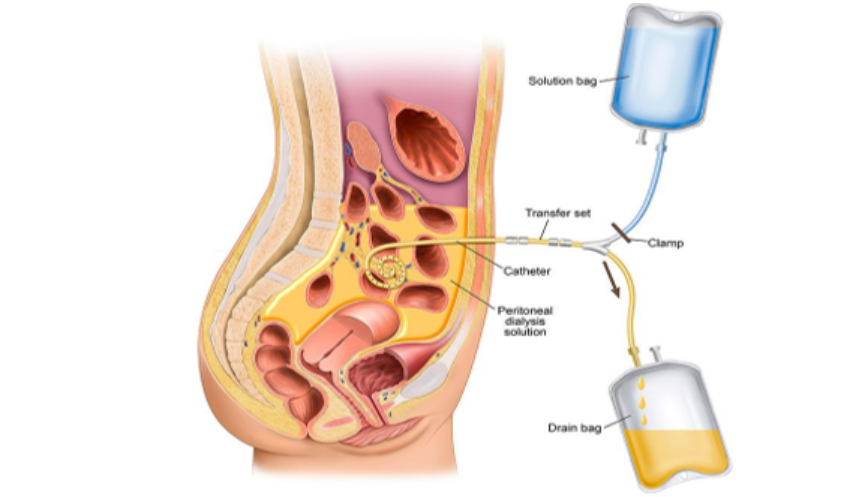
The peritoneum is a membrane that covers the abdominal organs and holds the organs in place. Peritoneal dialysis is a type of renal replacement therapy that uses the peritoneum as a filter to remove excess fluid and waste products from the body. The blood is cleaned from inside the body.
First, the patient undergoes a minor procedure whereby a peritoneal catheter (e.g., a Tenckhoff catheter) is inserted into the peritoneal cavity. Once the wound has healed and the catheter is ready for use, the patient can commence peritoneal dialysis.
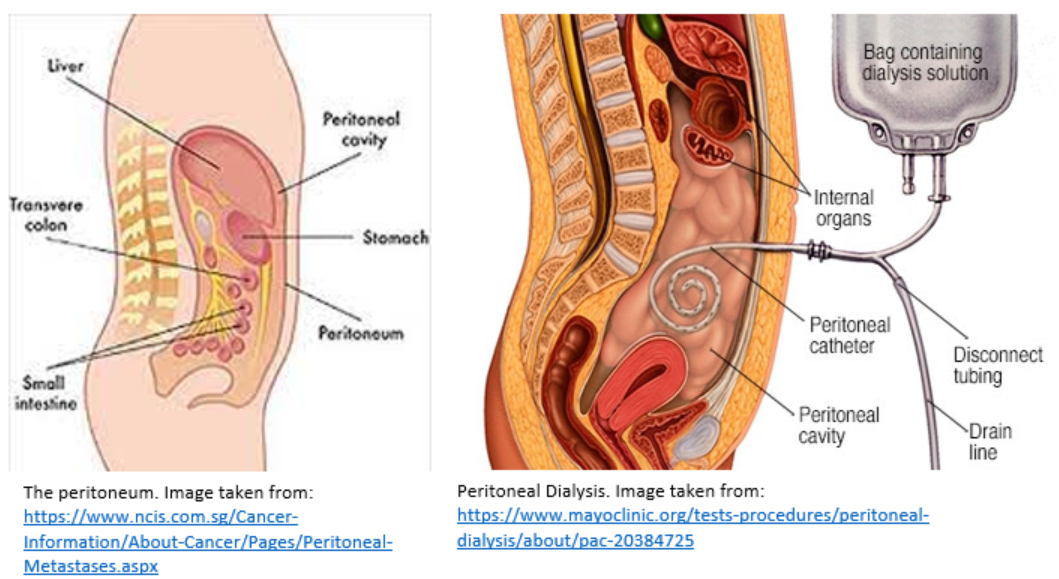
The catheter is used to administer a dialysis fluid (i.e., the dialysate) into the peritoneal cavity. This takes approximately 10 minutes. The blood itself stays in the blood vessels of the peritoneal membranes. The composition of the dialysate is specially formulated such that extra fluid and waste products are drawn out of these blood vessels and into the dialysate.
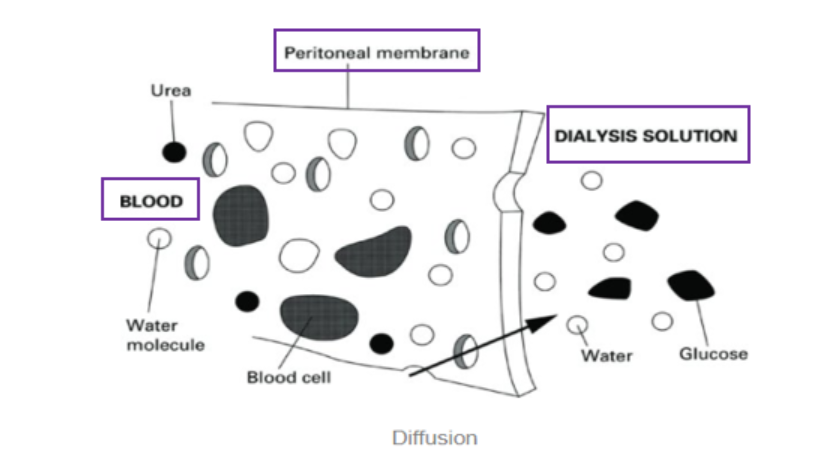
The exchange of solutes happens across the peritoneal membrane. As the peritoneal membrane lines the abdominal cavity AND the abdominal organs, it has a very large surface area. It is also highly vascular and semi-permeable, which makes it the perfect membrane for dialysis. Solutes and excess fluid move from peritoneal blood vessels into dialysate fluid by diffusion, convection, and osmosis.
- Diffusion: The passive transfer of small solute molecules (e.g., urea, creatinine, etc.) from an area of higher concentration (blood) to an area of lower concentration (the dialysate).
- Convection: Some solutes (e.g., those with a higher molecular weight) are transported by convection. This is a process whereby solutes are carried (‘dragged’) from one area to another by a flowing fluid.
- Osmosis: The diffusion of solvent (e.g., excess water/fluid) across the semi-permeable membrane. In peritoneal dialysis, the dialysate contains glucose which acts as the osmotic agent to drive fluid out of the blood and into the dialysate. That is, dialysate bags with higher glucose concentrations cause removal of more fluid from the patient. However, there is a risk of hyperglycaemia in diabetic patients, and as such there is the option of using dialysate bags that contain icodextrin instead of glucose. Icodextrin is a long-chain polymer that is less likely to be absorbed into the bloodstream compared to glucose.
 For a visual representation of movement across the semi-permeable peritoneal membrane, see the video below (starting at time 1.03 minutes). https://youtu.be/kBX4bD10MXM?t=63
For a visual representation of movement across the semi-permeable peritoneal membrane, see the video below (starting at time 1.03 minutes). https://youtu.be/kBX4bD10MXM?t=63
After a prescribed period of time (known as the ‘dwell time’), the fluid flows out of the peritoneal cavity into a drain bag. There are 2 different types of peritoneal dialysis, continuous ambulatory peritoneal dialysis (CAPD) and automated peritoneal dialysis (APD).
- In the case of Continuous Ambulatory Peritoneal Dialysis (CAPD), the patient performs their own exchanges 3-4 times throughout the day. The dialysate remains in the peritoneal cavity for 4-6 hours (or 8 hours overnight) before it is drained and discarded.
- In the case of Automated Peritoneal Dialysis (APD), the patient uses a machine to automate administration of the dialysate fluid and subsequent drainage. This type of dialysis is usually done overnight while the patient is sleeping. Multiple exchanges (3-7) can happen during this time.
| Advantages of Peritoneal Dialysis | Disadvantages of Peritoneal Dialysis |
| Blood never leaves the body | Infection (of skin around catheter exit site) |
| Can be done at home and scheduled around work, travel and other activities which preserves patient independence | Infection (of peritoneal fluid i.e, peritonitis) |
| Avoids the need for transport to a dialysis centre | Hernia |
| Easy and quick to learn | Weight gain |
| Needle-free | Burden on patient and carers |
| Preserves any residual renal function | Must be every day |
| Risk of membrane failing (and subsequent need to transition to haemodialysis) | |
| Cost of equipment |
In addition to the considerations outlined in the previous section on chronic kidney disease, there are some more considerations regarding the medication management of patients starting peritoneal dialysis:
- Peritoneal dialysis can manage acidotic complications of CKD. Therefore, for patients with CKD who are starting on peritoneal dialysis, oral sodium bicarbonate supplementation may be ceased.
- The dialysate fluid does not contain potassium. Therefore, patients starting on peritoneal dialysis can actually become hypokalaemic and so require oral potassium supplementation. You will remember that most other patients with CKD are at risk of hyperkalaemia. It will be most important to monitor serum potassium levels closely.
- Exit site (catheter site) cares. Skin contamination is a major cause of peritonitis and/or an exit site infection. Infection may necessitate removal of the catheter and thus cause patients to become dependent on another type of renal replacement therapy (e.g., haemodialysis or transplant). Patients will apply an antibiotic ointment to the site (e.g., mupirocin 2% ointment once a day usually after their evening shower).
- Prophylaxis of fungal peritonitis (e.g., with oral nystatin 500,000 units QID) should be considered for all patients requiring a course of any antibiotic, regardless of the indication for the antibiotic.
- Constipation must be avoided as straining can increase the risk of a hernia and associated damage to the peritoneal membrane. Constipation can also cause prolonged drainage times and/or difficulty draining the peritoneal fluid completely. Almost all patients will be on one (or more) aperients (laxatives).
Haemodialysis (HD)
Haemodialysis cleans a patient’s blood (via removal of excess fluid and waste products) from outside of their body, using a haemodialysis machine. Sometimes, haemodialysis is required short-term in the case of an acute kidney injury secondary to an acute condition such as sepsis. Other times, haemodialysis is required long-term (indefinitely) for patients with end-stage chronic kidney disease secondary to diabetes or hypertension, for example.
Haemodialysis is a complex process and patients are managed by a multidisciplinary team of healthcare professionals. If we also consider these patients in the context of their (often numerous) comorbidities, we can understand that haemodialysis patients are particularly big users of our healthcare services.
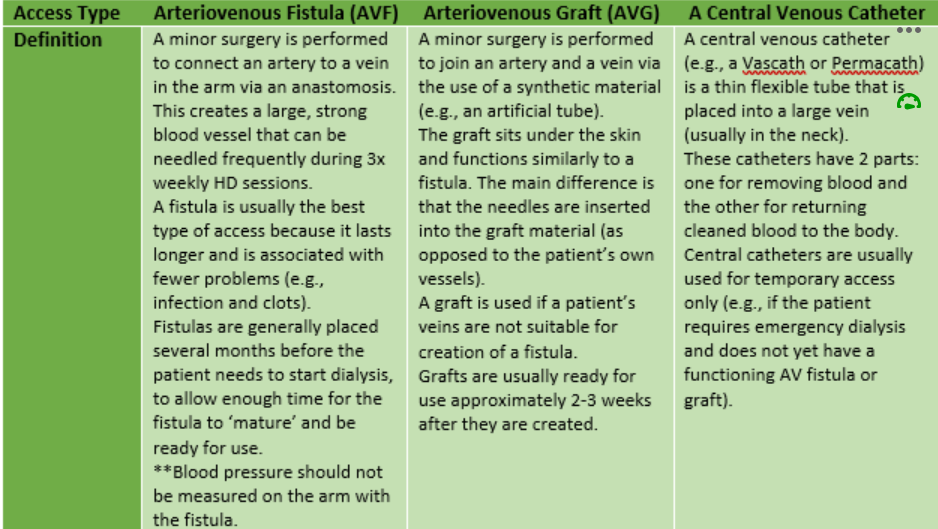
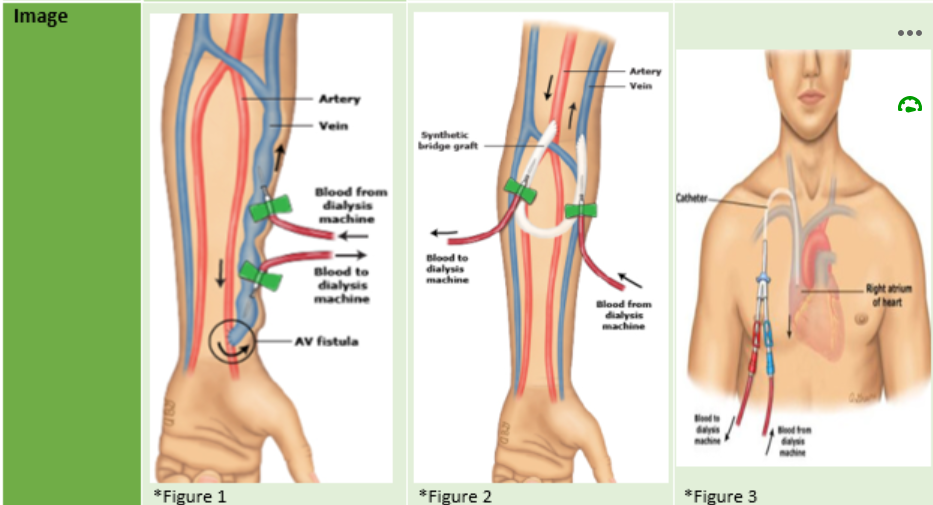
First, patients require vascular access. This is achieved by one of the following methods:
Most patients will travel to a haemodialysis centre 3 times per week for their dialysis session. Most commonly, patient bookings are scheduled for a morning or afternoon session on either:
- Monday, Wednesday & Friday, or
- Tuesday, Thursday & Saturday
The length of the dialysis session can be varied depending on the patient’s requirements. However, most sessions take 4-5 hours. You can understand that this is a significant time commitment for patients and so very few are able to maintain full time employment.
During the haemodialysis session, the patient is connected to the dialysis machine.
- The dialysate is pumped through the dialyser. Note: the electrolyte concentration of the dialysate may be altered depending on the patient’s individual requirements.
- At the same time, blood is being removed from the patient and pumped through the dialyser in the opposite direction compared to the dialysate. ‘Clean’ blood is then returned to the patient.
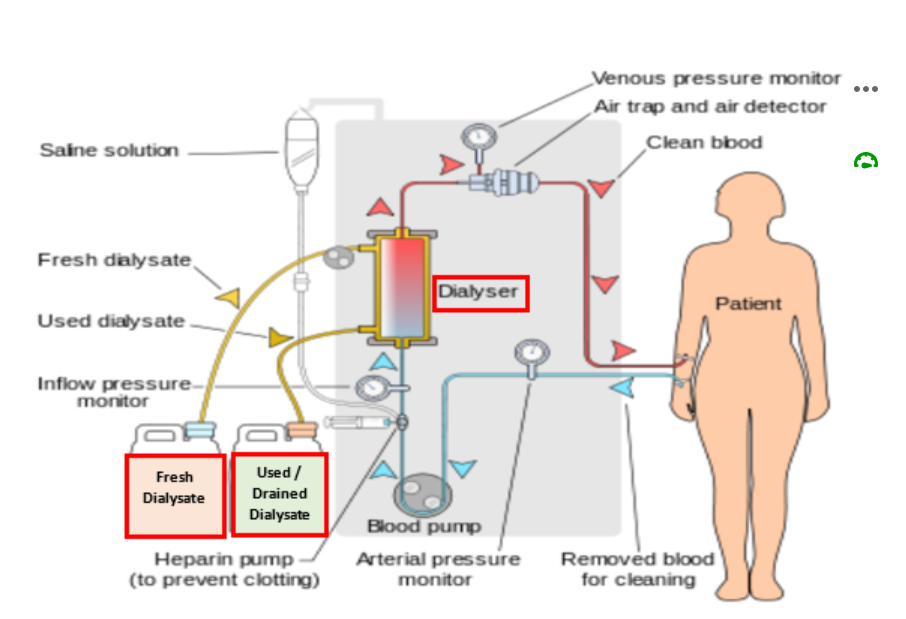
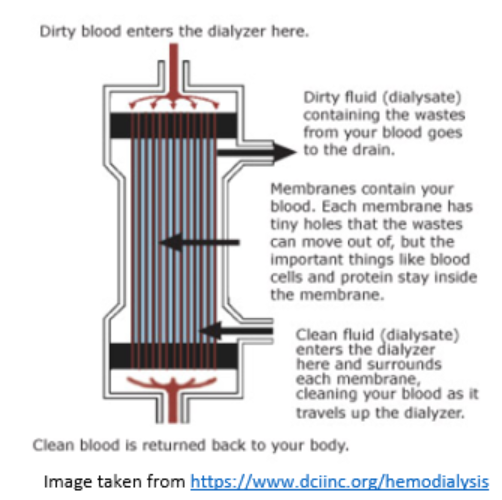
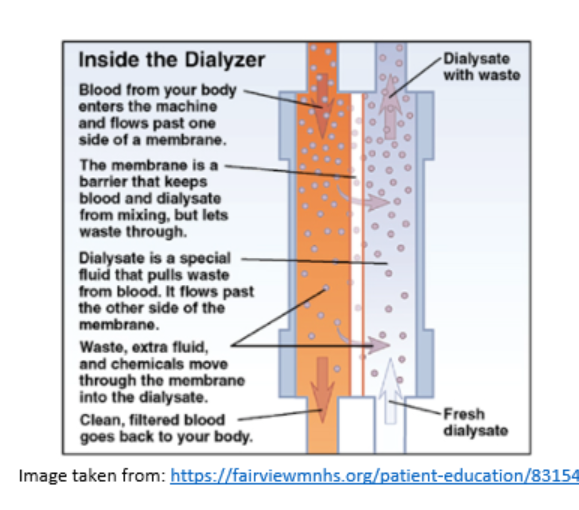
Inside the Dialyser
The dialyser is a plastic chamber which contains bundles of capillary tubes within which the blood circulates. Outside of these bundles, the dialysate circulates in the opposite direction.
Solutes and excess fluid move from the blood into the dialysate fluid by diffusion and ultrafiltration.
- Diffusion: The passive transfer of small solute molecules (e.g., urea, creatinine, etc.) from an area of higher concentration (blood) to an area of lower concentration (the dialysate).
- Ultrafiltration: The removal of fluid from the blood due to a driving pressure across the semipermeable membrane..
Watch the video at the link below for a summary of these key points.
https://www.freseniusmedicalcare.com/en/media/multimedia/videos/understanding-hemodialysis-video
Haemodialysis is more efficient than peritoneal dialysis at removing drugs from the circulation.
There are various physicochemical characteristics that influence the degree to which drugs are removed from the blood by haemodialysis.
- Molecular weight
- Water solubility
- Protein binding
- Volume of distribution
The type of dialysis membrane, blood flow rates, and dialysate flow rates also play a major role in determining the degree to which drugs are removed from the blood by haemodialysis.
In addition to the considerations outlined in the previous section on chronic kidney disease, there are some additional special considerations regarding the medication management of haemodialysis patients. These are not the same as for peritoneal dialysis patients.
Special considerations for haemodialysis patients include:
- Hypotension. This is a common complication of dialysis. We can understand this if we remember that haemodialysis involves the removal of blood from the body. When blood is removed from the body (albeit temporarily) blood pressure can decrease. If we don’t have enough blood pressure, then we aren’t able to maintain blood flow through the circuit and the patient at the same time. Often, this will mean that dialysis needs to be slowed or stopped altogether. As pharmacists, it is essential that we consider the timing of administration of antihypertensive drugs. We often need haemodialysis patients to withhold their antihypertensive medications in the morning on their dialysis days. It is important that patients are provided with education regarding the indications for each of their medications. For webster packed patients it is important that the packs reflect the plan to prevent hypotension during the dialysis session.
- Fluid balance. Haemodialysis patients will often have a fluid restriction and monitor their weights regularly. Sometimes patients gain too much fluid between dialysis sessions and so the dialysis ‘prescription’ (i.e., the dialysate composition, flow rates and/or session duration) requires modification in order to return the patient to their ‘dry weight’ (i.e., their ideal starting weight). Loop diuretics are only used if the patient is still producing some urine, in which case high doses are often required (e.g., furosemide 250mg BD (morning & midday)). For patients who are completely anuric, loop diuretics are ineffective and so should be ceased.
- Renal function cannot be estimated from any equation because creatinine levels fluctuate (i.e., creatinine levels are high prior to dialysis and decrease after dialysis). We assume that the GFR is less than 10ml/min. When checking and recommending drug doses, pharmacists should consult specialist renal drug dosing references, e.g., the Renal Drug Database, as the recommendations also differ depending on the type of dialysis the patient is receiving. Moreover, the timing of doses in relation to the haemodialysis session should be considered so that drugs that are readily dialysed off are administered after dialysis. Similarly, laboratory test results (e.g., for electrolyte levels) should be interpreted in relation to (1) the time the blood was taken and (2) the time of the haemodialysis session.
Finally, don’t forget the importance of liaison with other members of the multidisciplinary team.
- Haemodialysis necessitates major changes to a patient’s lifestyle. Social workers play a very important role in helping the patient to maintain social and emotional wellbeing.
- Dietitians also play a massive role in helping to optimise the nutritional status of patients with end stage renal failure on dialysis (PD or HD). For example, they can provide patients with advice about how to safely avoid phosphate-rich foods while still ensuring adequate protein intake.
Always consider the patient’s individual treatment goals and how these influence your treatment recommendations when you are working with patients who have been diagnosed with renal disease!!! It’s important to remember that the treatment goals will be very different for:
- A patient with CKD stage 3b, vs
- A patient who has recently commenced on peritoneal dialysis, vs
- An anuric patient who has been receiving haemodialysis for the past 5 years.
Renal Transplantation
Renal transplant is generally considered to be the preferred type of renal replacement therapy as it improves quality of life and can increase longevity. However, because patients with end-stage renal failure often have extensive comorbidities, only approximately 30% of patients are fit enough for both the transplant and the associated postoperative immunosuppression.
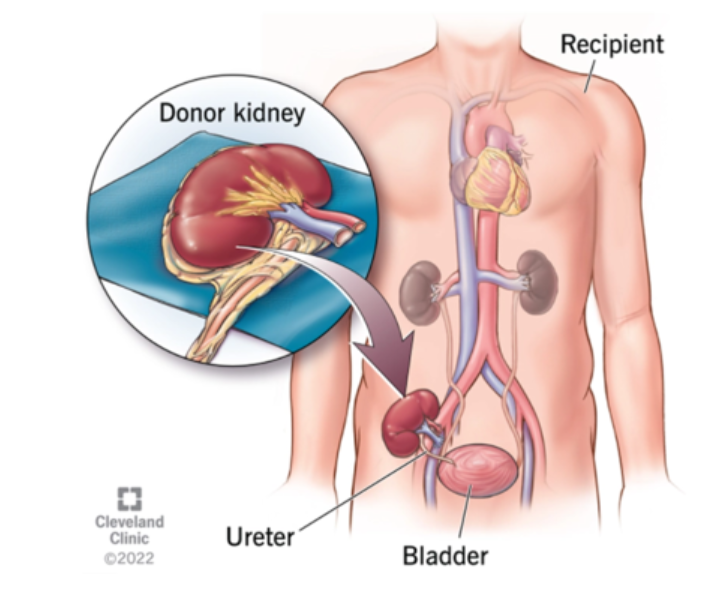
Only a kidney donated by a patient’s own identical twin would be genetically identical. In all other cases, the immune system recognises the transplanted kidney as ‘foreign’ and so attempts to destroy it. As such, patients require life-long immunosuppressive therapy to prevent organ rejection.
Immunosuppressive drug classes used to prevent organ rejection include:
- Corticosteroids
- Most commonly prednisolone/prednisone
- Antimetabolites
- Azathioprine
- Mycophenolate
- Calcineurin inhibitors
- Ciclosporin
- Tacrolimus
- Mammalian Target of Rapamycin (mTOR) Inhibitors
- Everolimus
- Sirolimus
- Biologic agents
- Antithymocyte globulins
- Basiliximab
There are many possible complications associated with immunosuppression to prevent organ rejection. These include:
- Cytomegalovirus (CMV)
- A common virus
- Rarely causes any problems in healthy people but can be fatal in immunosuppressed people
- The greatest risk is when:
- CMV-seronegative patients (R-) receive an organ from a CMV-seropositive donor (D+)
- CMV-seropositive patients (R+) receive T cell depleting antibodies
- Prophylaxis against CMV is prescribed according to local transplant centre procedure
- First line agent is valganciclovir (cytotoxic)
- Alternatives include ganciclovir (cytotoxic) and valaciclovir
- Duration of prophylaxis is generally 3-6 months following transplant
- Pneumocystis Jirovecii Pneumonia (PJP)
- Formerly known as Pneumocystis Carinii Pneumonia (PCP)
- A serious fungal infection of the lungs
- An opportunistic infection that affects immunosuppressed patients
- Prophylaxis against PJP is used in all kidney transplant recipients
- First line agent is trimethoprim with sulfamethoxazole
- Alternative agents include dapsone, atovaquone, and pentamidine (nebulised)
- Duration of prophylaxis can vary between transplant centres, but is generally lifelong
- Malignancy
Transplant medicine is complex.
- Patients should be on a combination of 3 immunosuppressants. A common combination is tacrolimus + mycophenolate +prednisolone. The combination regime is used (1) to target different parts of the immune system and (2) to enable each drug to be used at a relatively lower dose to help to prevent dose-related adverse effects (which can be life threatening!).
- It is very important to check that patients are adherent with their prescribed transplant medications to prevent rejection.
- Many transplant medications have a narrow therapeutic index and require therapeutic drug monitoring for dose optimisation.
- Many transplant medications also interact with other drugs. You should check for drug interactions every time you review a patient who has had any type of organ transplant in the past.
There is no lecture recording for this section.
Download the lecture notes here:
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING
This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.