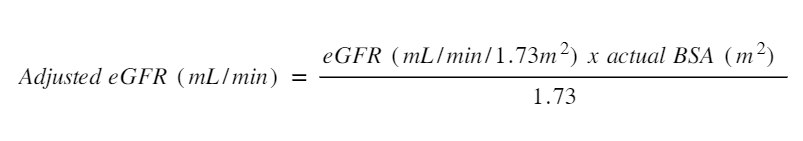4.2 Renal Anatomy and Physiology, Renal Function Tests and Dose Adjustments in Renal Impairment
Learning Outcomes
Be able to:
- Relate the anatomy of the renal system to its function
- Describe the mechanisms by which the renal system is regulated
- Predict the effect of physiological changes caused by drugs and disease on renal function.
Renal Anatomy and Physiology
 |
 |
 |
In chapter 2.1.4 we discussed different types of diuretics and their uses in clinical settings. We are now going to discuss the renal system in further detail. The kidneys filter around 200 liters of blood a day, most of which is reabsorbed (a typical blood volume for an adult is around 4500-5700 m). The purpose of the kidneys is to remove toxins, metabolic waste and excess electrolytes from the blood, maintain an appropriate balance between fluid and salts, maintain blood pH, metabolise vitamin D to its active form, undergo gluconeogenesis during fasting and produce hormones such as Renin to regulate blood pressure and erythropoietin to stimulate red blood cells.
📺 Watch the video on renal anatomy and physiology (1:06 minutes)
Blood supply to the kidneys – big renal artery to tiny glomerular capillaries
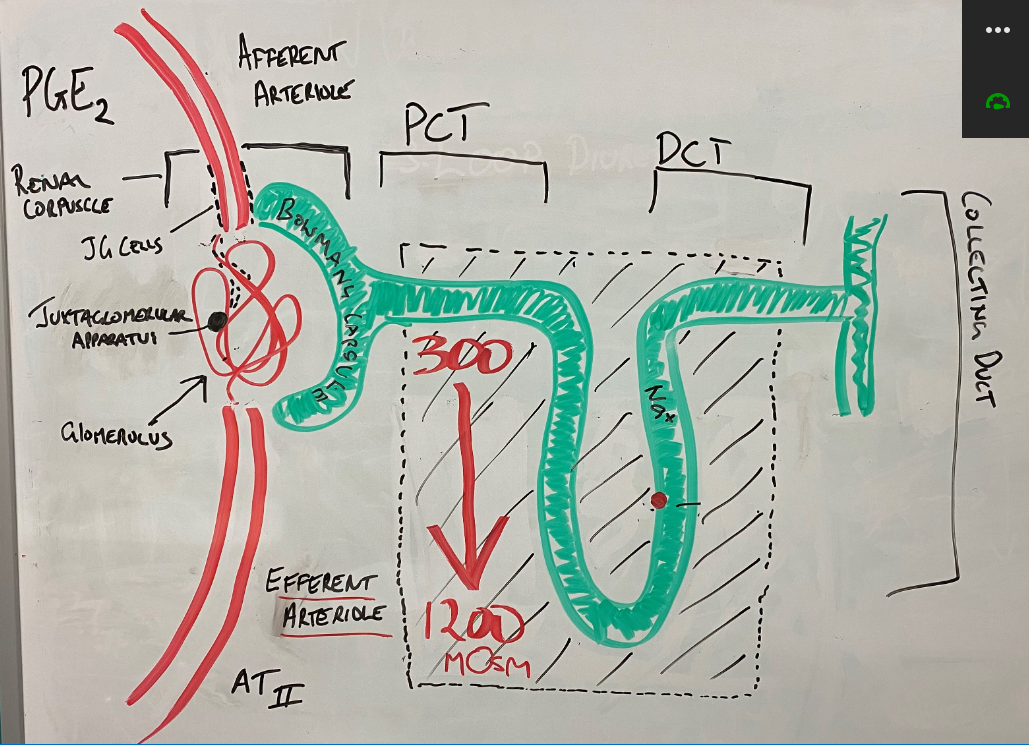
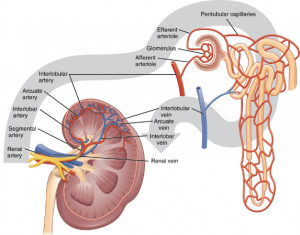
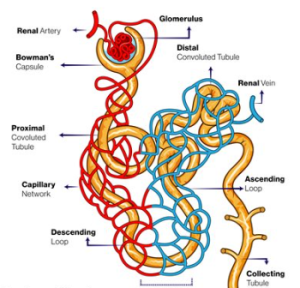
The aorta supplies blood to the renal arteries, which branch into ever increasingly smaller arteries until they eventually form the afferent arteriole. The afferent arteriole turns into the glomerulus – a series of very fine capillaries in the renal corpuscle that form the main interface between the blood supply and the glomerular (AKA Bowman’s) capsule and the broader structure known as the nephron.
Hydrostatic pressure is the main influencer of filtrate volume
The endothelium of the glomeruli capillary is fenestrated making them porous to fluid and solutes. The fenestrations are large enough to allow large amount of solute-rich filtrate to pass into the glomerular capsule but small enough to block the movement of important proteins like albumin, globulins and fibrinogen into the filtrate. The rate of filtrate production is (in a normal healthy person) dependent upon the hydrostatic pressure at the glomerulus (HPg). The higher the hydrostatic pressure, the higher the volume of filtrate… the lower the hydrostatic pressure, the lower the volume of filtrate. You can use the term filtrate and urine interchangeably.
PGE2, renin & angiotensin II and blood volume significantly influence glomerular hydrostatic pressure
The juxtaglomerular (JG) cells are mechanoreceptors capable of sensing blood pressure in the afferent arteriole. In response to lower blood volume (let’s call low blood volume and low blood pressure the same thing eh!) in the afferent arteriole, the JG cells release renin which eventually (as part of the Renin-Angiotensin-Aldosterone System (RAAS) cause the production of angiotensin II (AT-II) – a potent vasoconstrictor. AT-II has significant vasoconstrictor effects on the arterioles (as well as stimulating sympathetic nervous stimulation, increasing aldosterone release (and sodium reabsorption), and increasing the release of anti-diuretic hormone (ADH – aka vasopressin) from the pituitary gland. It has little effect on the afferent arteriole but does cause vasoconstriction of the efferent arteriole.
PGE2 (PGI2 is the other main PG) is the main renal prostaglandin responsible for dilatation of the afferent arteriole. Prostaglandin E2 is the product of the metabolism of arachidonic acid by cyclooxygenase I and II (COX I & COX II), in response to decreased actual or effective circulating volume. This results in greater blood flow to the glomerulus. PGE2 also stimulates the formation of AT-II. The combined effect of the vasodilation of the afferent arteriole by PGE2 and vasoconstriction of the efferent arteriole by AT-II results in raised hydrostatic pressure (HPg) and an increased glomerular filtration rate.
📺Watch the vodcast on regulation of glomerular filtration and glomerular hydrostatic pressure (5.20 minutes)
The nephron is made up of the renal corpuscle (consisting of the glomerulus, juxtaglomerular apparatus and Bowman’s (glomerular) capsule. This apparatus is responsible for the filtration of the blood and the formation of filtrate in the renal nephron.
The renal corpuscle joins with the proximal convoluted tubule. It is the proximal convoluted tubule that is highly permeable to water and is therefore most active in fluid reabsorption. It reabsorbs 65% of the water and sodium, all of the glucose, lactate and amino acids and 55-60% of the chloride and potassium ions. This all occurs before the filtrate reaches the descending loop of Henle.
The descending limb of the Loop of Henle (LoH) is highly permeable to water but relatively impermeable to sodium and other solutes – this is contrasted to the thick ascending portion of the LoH which is relatively impermeable to water but actively transports sodium ions via the Na+/K+ATPase pump creating an increasing sodium concentration gradient from the outer cortex to the deepest part of the medulla. This concentration gradient starts at around 300 mOsm and gets as high as 1200 mOsm. This concentration gradient forms the basis of the counter-current multiplier system and explains the powerful absorptive properties of the renal nephron. The Loop of Henle is responsible for around 25% of sodium reabsorption.
The distal convoluted tubule and collecting duct is the last section of the renal nephron. Around 10% of the sodium and 25% of the water remain in the tubule. The distal convoluted tubule reabsorbs around 5% of the total original sodium concentration via a Na+/Cl- symporter. The collecting duct is typically relatively impermeable to water in the absence of antidiuretic hormone (ADH). ADH causes the creation of water channels called aquaporin’s. This coupled with the release of aldosterone (under the influence of angiotensin-II), up-regulate the expression of Na+/K+ ATPases which increase sodium reabsorption. This accounts for up to 3% of sodium reabsorption in the nephron. With the help of ADH induced aquaporins, aldosterone fine tunes the final volume of filtrate and final volume of water and sodium reabsorption.
📺Watch the vodcast on the function of the nephron (3.15 minutes)
Learning Outcomes
Be able to:
- Describe the implications of changes in the different laboratory tests used to evaluate organ function (briefly)
- Explain the specificity of each of the tests for organ disease and use patterns of results to make determinations about specificity and renal and hepatic function.
Chronic kidney disease sometimes requires drug dose adjustments. The prevalence of chronic kidney disease is increasing meaning pharmacists will increasingly come into contact with patients with varying degrees of chronic kidney dysfunction. There are equal risks in providing inappropriately high drug doses in patients with chronic renal impairment as there is with the inappropriate reduction of dose resulting in sub-therapeutic disease management. To appropriately provide advice on dose adjustment, an understanding of the different renal function tests and the drugs that require adjustment in chronic renal impairment is important.
📺Watch the following YouTube introductory video on renal function tests. Watch the first 4.15 minutes. (4.15 min.)
Glomerular filtration rate
Glomerular filtration is a passive process where solute and fluid is forced across the membrane. Small molecules less than 3 nm in diameter and water pass freely across the membrane taking urea, creatinine, sodium, potassium, chloride, calcium, glucose and other small molecule with 180-200 litres of water a day into the nephron as filtrate (urine). Larger molecules such as red blood cells and proteins larger than 5 nm are too large to pass through the fenestrated membrane of the glomerulus and remain in the blood. The retention of the large plasma proteins assist in maintaining the colloid osmotic pressure. It is useful to know creatinine is not just cleared via the glomerulus but also by active secretion into the nephron tubules. This may result in estimates of GFR higher than the true measured GFR (mGFR) (The mGFR is determined after giving an exogenous filtration marker, such as 51Cr-EDTA, 125I-iothalamate, DTPA or MAG3. It is the most reliable method of quantifying GFR because these markers are filtered and not substantially secreted into or reabsorbed from the nephron.)
eGFR
Glomerular filtration rate gives an approximate of the amount of blood being filtered by the kidneys per minute. An estimation of glomerular filtration can be made using several equations – a common one in Australia is the newer CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula and the previously commonly used equation MRDR(4V) (4-variable Modification of Diet in Renal Disease). These equations use a combination of serum creatinine level, age, body size and gender to estimate GFR. eGFR, like the Cockcroft-Gault equation typically overestimates measured GFR due to variability in actual body surface area in patients and differences between actual and real body weight.
So, we must remember eGFR is only an estimation of kidney function but while not precise, it is used as an affordable estimate of renal function (the more accurate mGFR is very expensive to conduct). The eGFR is roughly equivalent to the percentage of renal function. So, a young person with an eGFR of 95ml/min/1.73m2 has roughly 95% kidney function in relation to the estimated kidney function for a person of that age, sex and body surface area (and sometimes weight, height and race). A person with a eGFR of 15ml/min/1.73m2 has roughly 15% kidney function. It is not exact but a useful trick to quickly get an association between the reported number and degree of renal impairment. The eGFR can be used to estimate the state of kidney disease – a useful measure when speaking generally about a patient’s renal function.
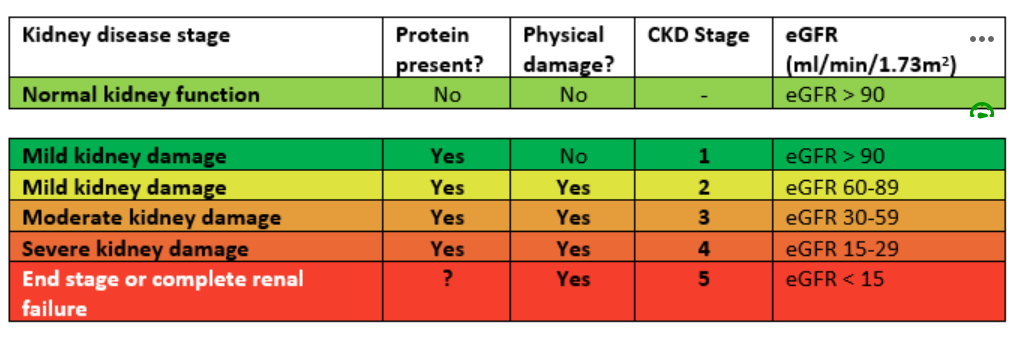 eGFR is a screening tool for chronic kidney disease but can also be used to make adjustments to dose. One of the limitations of using eGFR is the assumptions around the ‘average’ body surface area (BSA) and using this assumption in the extremes of height and weight. The eGFR is reported for someone with a BSA of 1.73m2. Most people are closer to 1.9 to 2.0 m2. This means the eGFR should be adjusted (or de-indexed) before being used to make recommendations on dose adjustment.
eGFR is a screening tool for chronic kidney disease but can also be used to make adjustments to dose. One of the limitations of using eGFR is the assumptions around the ‘average’ body surface area (BSA) and using this assumption in the extremes of height and weight. The eGFR is reported for someone with a BSA of 1.73m2. Most people are closer to 1.9 to 2.0 m2. This means the eGFR should be adjusted (or de-indexed) before being used to make recommendations on dose adjustment.
Calculating renally cleared drug dose adjustment using the Cockcroft and Gault (1979) formula
The use of eGFR assists in determining the degree of renal dysfunction. The Cockcroft and Gault (1979) formula provides 3 categories for determining the need and extent of dose adjustment for patients with renal impairment. The calculation is not useful in determining renal function for the purpose of categorising chronic renal disease.
The Cockcroft and Gault formulation uses serum creatinine and ideal or actual body weight (whichever is lower) and is adjusted based on sex (multiple x 0.85 for females). The Cockcroft and Gault formula for the determination of dose adjustment is found below. You are no doubt familiar with this equation already and remember that ideal body weight can be calculated using the following formula:
- Females: 45.5kg + 0.9kg/cm for each cm > 152cm.
- Males: 50kg + 0.9kg/cm for each cm > 152cm.

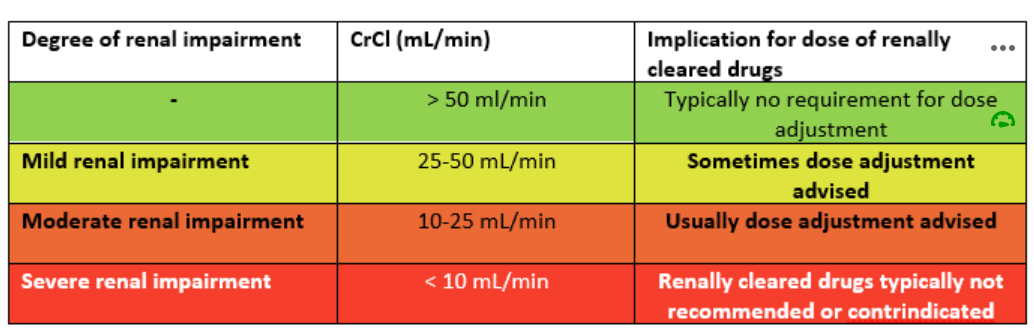
The definitions of the 3 degrees of renal impairment for the purpose of dose adjustment of drugs cleared by the kidneys are as follows:
The Cockcroft-Gault equation and eGFR are not used interchangeably and both have their role in patients with renal dysfunction.
📺Watch the vodcast on the relevance of eGFR and the Cockcroft-Gault equation (8.35 mins)
Clinical references for determining dose adjustment
The two most commonly used drug indexes used to provide information on dose adjustment are the AMH and Therapeutic Guidelines. Of the two, the AMH provides more comprehensive advice. You should familiarise yourself with prescribing in renal impairment in your AMH.
What drugs commonly need dose adjustment in renal impairment?
A number of drug classes require dose adjustment consideration in chronic renal impairment. Commonly prescribed drugs that require dose adjustment in renal impairment include:
- Antiviral drugs
- Hypoglycaemic drugs such as metformin, sulfonylureas and insulin
- Antibiotics such as aminoglycosides, vancomycin, carbapenems, penicillamine (and many others)
- Anticoagulants including low molecular weight heparin
- cardiac drugs like digoxin, sotalol and atenolol
- Diuretics – particularly at low CrCl rates
- Opioids
- Anticonvulsants
- Drugs for gout like colchicine and allopurinol
- Methotrexate
- Lamivudine.
📚 Read/Explore
- Listen to the podcast on dose adjustment and chronic kidney disease by Assoc. Prof. Darren Roberts 🔗 Available here.
- Read the following article on how to adjust drug doses in chronic kidney disease: Stefani, M., Singer, R. & Roberts, D. How to adjust drug doses in chronic kidney disease. Australian Prescriber; 2019;42:163-7. 🔗 Download here
- Activity
- Three patients have had their renal function tests returned. Find a eGFR calculator or got to https://kidney.org.au/health-professionals/egfr-calculator and calculate the eGFR for the following 3 cases:
- A healthy 27 year old male with serum creatinine 94 micromoles/L
- A healthy 70 year old female with serum creatinine 82 micromoles/L
- An unwell 34 year old Indigenous male with diabetes with serum creatinine 330 micromoles/L
- Three patients have had their renal function tests returned. Find a eGFR calculator or got to https://kidney.org.au/health-professionals/egfr-calculator and calculate the eGFR for the following 3 cases:
- Activity
- Answer the following questions for the 3 patients above.
- What is the approximate renal function of each of the patients?
- Why are the first two cases so different? (that is, what are the parameters that change their eGFR result)?
- Why are the first and last case so different – what initial conclusion can you draw?
- Answer the following questions for the 3 patients above.
- Activity
- Calculate the following BSAs using this link
- Case 1: calculate your own BSA using your own height and weight
- Case 2: calculate a BSA for a 130kg male with a height of 178cm
- Case 3: calculate a BSA for a 50kg female with a height of 163cm.
- The three different actual BSA’s will make a difference to the eGFR and may lead to inappropriate dose adjustments unless the de-indexed method is used. Calculate an adjusted or de-indexed eGFR for these patients assuming a reported eGFR of 60 mL/min/1.73m2. Would a non-adjusted eGFR provide an overestimate, underestimate or accurate estimate? Typically, does eGFR overreport or under report actual eGFR for obese patients and underweight patients?
- Calculate the following BSAs using this link
- Activity
- Calculate your own ideal body weight and that of someone in your family.
- Activity
- Three patients are prescribed allopurinol. The normal dose for gout is 300mg once daily. Calculate the dose adjustment based on the following patient parameters:
- A healthy 27 year old male with serum creatinine 94 micromoles/L, weight 85kg
- A healthy 70 year old female with serum creatinine 82 micromoles/L, weight 52kg
- An unwell 34 year old Indigenous male with diabetes with serum creatinine 330 micromoles/L, weight 67kg
- Note: To convert μmol/l to mg/dl, multiply by 0.0113. To convert mg/dl to μmol/l, multiply by 88.4.
- Three patients are prescribed allopurinol. The normal dose for gout is 300mg once daily. Calculate the dose adjustment based on the following patient parameters:
- Read the following article on prescribing in renal disease: Faull, R. & Lee, L. Prescribing in renal disease. Australian Prescriber; 2007;30:17-20. 🔗Download here. Read the section on dose alteration in renal impairment.
Download the lecture notes here:
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING
This material has been reproduced and communicated to you by or on behalf of James Cook University in accordance with section 113P of the Copyright Act 1969 (Act).
The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.