4. Water biology

What will I be able to do at the end of this chapter?
- Explain the properties of water that make it essential to life
- Distinguish between acids and bases and explain their role in pH
- Discuss the role of buffers in helping the body maintain pH homeostasis.
The concepts you learned about in Chapter 3: Chemistry for life govern all forms of matter and would work as a foundation for geology as well as biology. We now narrow the focus to the chemistry of human life; that is, the compounds important for the body’s structure and function. In general, these compounds are either inorganic or organic.
This chapter examines the three groups of inorganic compounds essential to life:
- water
- salts
- acids and bases
4.1 | Why is water important for life?
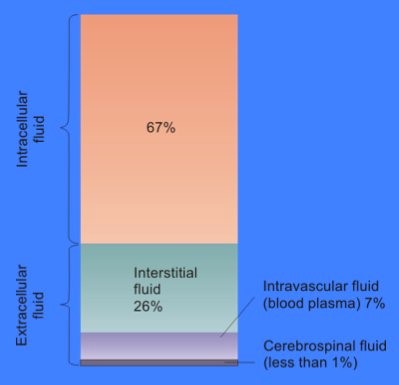
As much as 70 percent of an adult’s body weight is water. Most of this water is contained within the cells (intracellular), the rest being extracellular (Figure 4.2). Water has several properties that make it indispensable to human functioning.
Water as a lubricant and cushion
Water is a major component of many of the body’s lubricating fluids. Just as oil lubricates the hinge on a door, water in synovial fluid lubricates the actions of body joints, and water in pleural fluid (the fluid between the layers of membranes, or pleura, which surround the lung) helps the lungs expand and recoil with breathing. Watery fluids help keep food flowing through the digestive tract and ensure that the movement of adjacent abdominal organs is friction free.
Water also protects cells and organs from physical trauma, cushioning the brain within the skull, for example, and protecting the delicate nerve tissue of the eyes. Water cushions a developing foetus in the mother’s womb as well.
Water as a heat sink
A heat sink is a substance or object that absorbs and dissipates heat but does not experience a corresponding increase in temperature. In the body, water absorbs the heat generated by chemical reactions without greatly increasing in temperature. Moreover, when the environmental temperature soars, the water stored in the body helps keep the body cool. This cooling effect happens as warm blood from the body’s core flows to the blood vessels just under the skin and is transferred to the environment. At the same time, sweat glands release warm water in sweat. As the water evaporates into the air, it carries away heat, and then the cooler blood from the periphery circulates back to the body core.
Water as a component of liquid mixtures
A mixture is a combination of two or more substances, each of which maintains its own chemical identity. In other words, the constituent substances are not chemically bonded into a new, larger chemical compound. The concept is easy to imagine if you think of powdery substances such as flour and sugar; when you stir them together in a bowl, they obviously do not bond to form a new compound. The room air you breathe is a gaseous mixture, containing three discrete elements—nitrogen, oxygen, and argon—and one compound, carbon dioxide. There are three types of liquid mixtures, all of which contain water as a key component. These are solutions, colloids, and suspensions.
Solutions
For cells in the body to survive, they must be kept moist in a water-based liquid called a solution. In chemistry, a liquid solution consists of a solvent (in this case water) that dissolves a substance called a solute.
Solution = Solvent + Solute
An important characteristic of solutions is that they are homogeneous; that is, the solute molecules are distributed evenly throughout the solution. If you were to stir a teaspoon of sugar into a glass of water, the sugar would dissolve into sugar molecules separated by water molecules. The ratio of sugar to water in the left side of the glass would be the same as the ratio of sugar to water in the right side of the glass. If you were to add more sugar, the ratio of sugar to water would change, but the distribution—provided you had stirred well—would still be even.
Water is considered the “universal solvent” and it is believed that life cannot exist without water because of this. Water is certainly the most abundant solvent in the body; essentially all of the body’s chemical reactions occur among compounds dissolved in water. Because water molecules are polar, with regions of positive and negative electrical charge, water readily dissolves ionic compounds and polar covalent compounds. Such compounds are referred to as hydrophilic, or “water-loving.” As mentioned above, sugar dissolves well in water. This is because sugar molecules contain regions of hydrogen-oxygen polar bonds, making it hydrophilic. Nonpolar molecules, which do not readily dissolve in water, are calledhydrophobic, or “water-fearing.” This is important when we come to look at how substances cross the cell membrane – which is also hydrophobic.
Colloids and suspensions
Just for completeness, we will take a quick look at colloids and suspensions. A colloid is a mixture that is somewhat like a heavy solution. The solute particles consist of tiny clumps of molecules large enough to make the liquid mixture opaque (because the particles are large enough to scatter light). Familiar examples of colloids are milk and cream. In the thyroid glands, the thyroid hormone is stored as a thick protein mixture also called a colloid.
A suspension is a liquid mixture in which a heavier substance is suspended temporarily in a liquid, but over time, settles out. This separation of particles from a suspension is called sedimentation. An example of sedimentation occurs in the blood test that establishes erythrocyte sedimentation rate, or ESR. The test measures how quickly red blood cells in a test tube settle out of the watery portion of blood over a set period of time. Rapid sedimentation of blood cells does not normally happen in the healthy body, but aspects of certain diseases can cause blood cells to clump together, and these heavy clumps of blood cells settle to the bottom of the test tube more quickly than do normal blood cells.
A special property of water
Polarity is something we touched on briefly in the last chapter, when we looked at the difference between polar covalent molecules, and non-polar covalent molecules. In polar molecules where hydrogen is part of the molecule, a relatively weak bond, called a hydrogen bond can form between the hydrogen of one atom (which is slightly positive) and the slightly negative part of another atom.
The most common example of hydrogen bonding in the natural world occurs between molecules of water. It happens before your eyes whenever two raindrops merge into a larger bead, or a creek spill into a river. Hydrogen bonding occurs because the weakly negative oxygen atom in one water molecule is attracted to the weakly positive hydrogen atoms of two other water molecules (Figure 4.3).
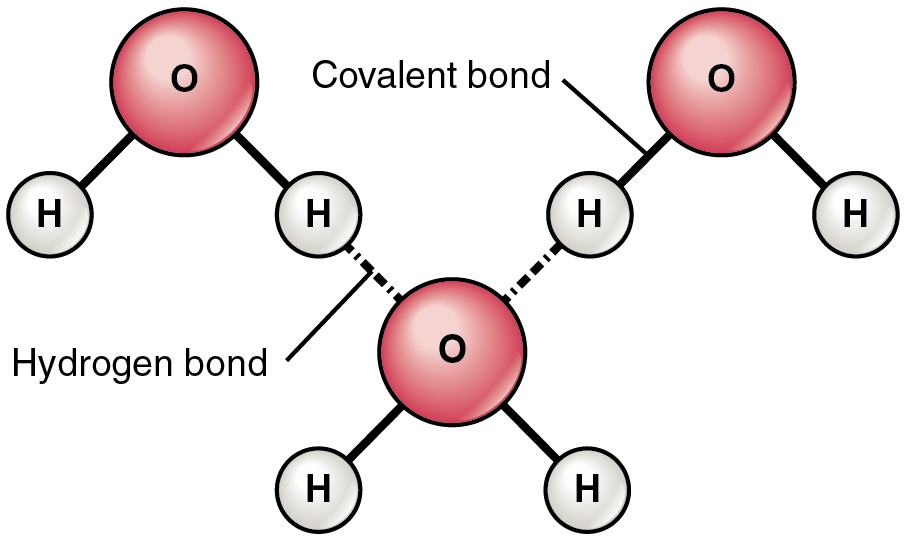
Water molecules also strongly attract other types of charged molecules as well as ions. This explains why table salt, for example, which consists of equal numbers of positively-charged sodium (Na+) and negatively-charged chloride (Cl–), dissolves so readily in water. This happens because hydrogen bonds form between the water molecules and the electrically charged ions Na+ and Cl–.
Water molecules also repel molecules with nonpolar covalent bonds, like fats, lipids, and oils. You can demonstrate this with a simple kitchen experiment: pour a teaspoon of vegetable oil, a compound formed by nonpolar covalent bonds, into a glass of water. Instead of instantly dissolving in the water, the oil forms a distinct bead because the polar water molecules repel the nonpolar oil. In the next chapter, we will discuss the properties of water at greater length.
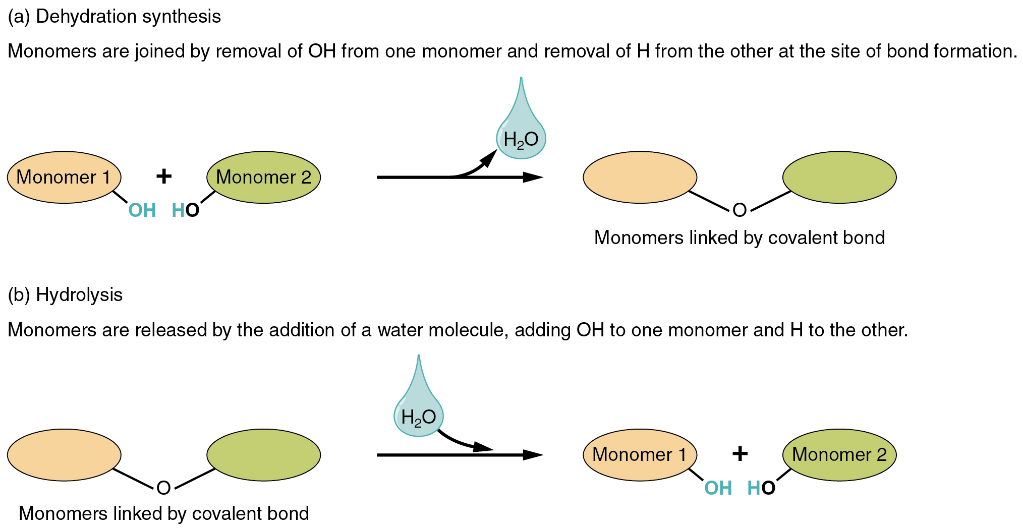
Water as a part of chemical reactions
In addition to providing the location for chemical reactions to occur and interacting with polar and charged particles, there are two types of chemical reactions that involve water directly. One involves the creation of water: dehydration synthesis (or condensation reaction), while the other involves the consumption of water: hydrolysis (Figure 4.4 b).
These reactions are reversible and play an important role in the chemistry of organic compounds (which will be discussed later in the textbook).
How is water distributed throughout the body?
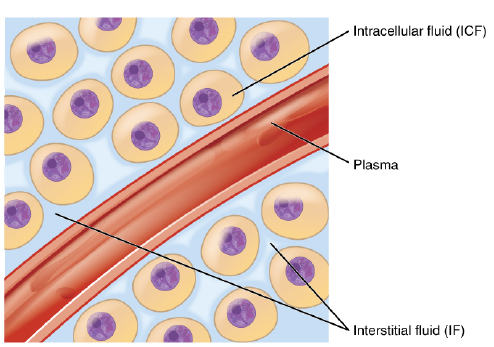
Body fluids can be discussed in terms of their specific fluid compartment, a location that is largely separate from another compartment by some form of a physical barrier. The intracellular fluid (ICF) compartment is the system that includes all fluid enclosed in cells by their plasma membranes. Extracellular fluid (ECF) surrounds all cells in the body. Extracellular fluid has two primary constituents: the fluid component of the blood (called plasma) and the interstitial fluid (IF) that surrounds all cells not in the blood (Figure 4.5).
Intracellular fluid
The ICF lies within cells and is the principal component of the cytosol/cytoplasm. The ICF makes up about 60 percent of the total water in the human body, and in an average-size adult male, the ICF accounts for about 25 litres (seven gallons) of fluid (Figure 4.2). This fluid volume tends to be very stable, because the amount of water in living cells is closely regulated. If the amount of water inside a cell falls to a value that is too low, the cytosol becomes too concentrated with solutes to carry on normal cellular activities; if too much water enters a cell, the cell may burst and be destroyed.
Extracellular fluid
The ECF accounts for the other one-third of the body’s water content. Approximately 20 percent of the ECF is found in plasma. Plasma travels through the body in blood vessels and transports a range of materials, including blood cells, proteins (including clotting factors and antibodies), electrolytes, nutrients, gases, and wastes. Gases, nutrients, and waste materials travel between capillaries and cells through the IF. Cells are separated from the IF by a selectively permeable cell membrane that helps regulate the passage of materials between the IF and the interior of the cell.
The body has other water based ECF. These include the cerebrospinal fluid that bathes the brain and spinal cord, lymph, the synovial fluid in joints, the pleural fluid in the pleural cavities, the pericardial fluid in the cardiac sac, the peritoneal fluid in the peritoneal cavity, and the aqueous humour of the eye. As these fluids are outside of cells, they are also considered components of the ECF compartment. We will talk more about the movement of water and solutes between the different fluid compartments in Chapter 5: Membrane transport.
4.2 | What are salts and electrolytes?
Salts are formed when ions form ionic bonds, the example that we looked at in the last chapter was Na+ and Cl–, which together form the salt sodium chloride. In these reactions, one atom gives up one or more electrons, and thus becomes positively charged, whereas the other atom accepts one or more electrons and becomes negatively charged. What happens when a salt dissolves in water? The answer is that the negative and positive ions dissociate. You can now define a salt as a substance that, when dissolved in water, dissociates into ions other than H+ or OH–. This fact is important in distinguishing salts from acids and bases, discussed next.
A typical salt, NaCl, dissociates completely in water (Figure 4.6). The positive and negative regions on the water molecule (the hydrogen and oxygen ends respectively) attract the negative chloride and positive sodium ions, pulling them away from each other. In solution, therefore, salts dissociate into ions. These ions are electrolytes; they are capable of conducting an electrical current in solution; this property is critical to the function of ions in transmitting nerve impulses and prompting muscle contraction.
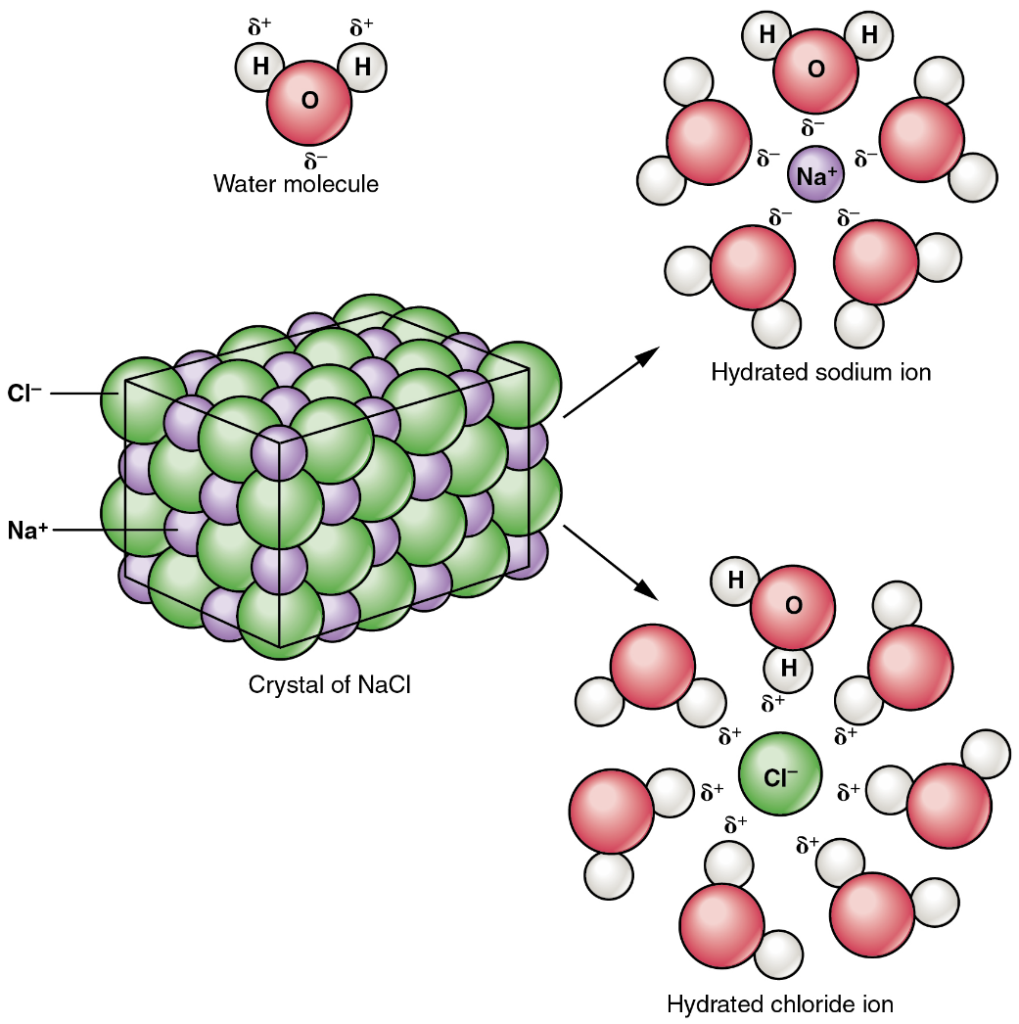
The body contains a large variety of ions, or electrolytes, which perform a variety of functions. Some ions assist in the transmission of electrical impulses along cell membranes in neurons and muscles. Other ions help to stabilise protein structures in enzymes. Still others aid in releasing hormones from endocrine glands. All of the ions in plasma contribute to the osmotic balance that controls the movement of water between cells and their environment.
Electrolytes in living systems include sodium, potassium, chloride, bicarbonate, calcium, phosphate, magnesium, copper, zinc, iron, manganese, molybdenum, copper, and chromium. In terms of body functioning, six electrolytes are most important: sodium, potassium, chloride, bicarbonate, calcium, and phosphate.
Table 4.1 lists the reference values for blood plasma, cerebrospinal fluid (CSF), and urine for the six ions addressed in this section. In a clinical setting, sodium, potassium, and chloride are typically analysed in a routine urine sample. In contrast, calcium and phosphate analysis requires a collection of urine across a 24-hour period, because the output of these ions can vary considerably over the course of a day. Urine values reflect the rates of excretion of these ions. Bicarbonate is the one ion that is not normally excreted in urine; instead, it is conserved by the kidneys for use in the body’s buffering systems.
| Name | Chemical symbol | Plasma | CSF | Urine |
| Sodium | Na+ | 136.00-146.00(mM) | 138.00-150.00(mM) | 40.00-220.00(mM) |
| Potassium | K+ | 3.50-5.00(mM) | 0.35-3.5(mM) | 25.00-125.00(mM) |
| Chloride | Cl– | 98.00-107.00(mM) | 118.00-132.00(mM) | 1110.00-250.00(mM) |
| Bicarbonate | HCO3 – | 22.00-29.00(mM) | — | — |
| Calcium | Ca2+ | 2.15-2.55(mmol/day) | — | up to 7.49(mmol/day) |
| Phosphate | HPO4 2- | 0.81-1.45(mmol/day) | — | 12.90-42.00(mmol/day) |
4.3 | What are acids, bases and buffers?
Acids
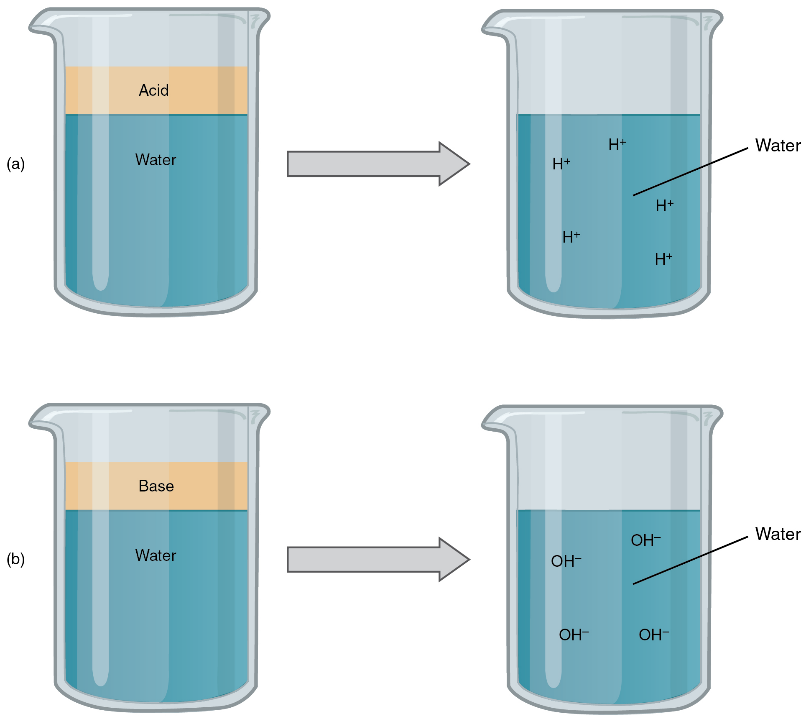
Acids and bases, like salts, dissociate in water into electrolytes. Acids and bases can very much change the properties of the solutions in which they are dissolved. An acid is a substance that releases hydrogen ions (H+) in solution (Figure 4.7a). Because an atom of hydrogen has just one proton and one electron, and the single electron is missing in the H+, the hydrogen ion is simply just a proton. This solitary proton is highly likely to participate in chemical reactions. Strong acids are compounds that release all of their H+ in solution; that is, they ionise completely. Hydrochloric acid (HCl), which is released from cells in the lining of the stomach, is a strong acid because it releases all of its H+ in the stomach’s watery environment. This strong acid aids in digestion and kills ingested microbes. Weak acids do not ionise completely; that is, some of their hydrogen ions remain bonded within a compound in solution. An example of a weak acid is vinegar or acetic acid; it is called acetate after it gives up a proton.
Bases
A base (also called an alkali) is a substance that releases hydroxyl ions (OH–) in solution (see Figure 4.7b), or one that accepts H+ already present in solution. The OH– combine with H+ present to form a water molecule, thereby removing H+ and reducing the solution’s acidity. Strong bases release most or all of their hydroxyl ions; weak bases release only some hydroxyl ions or absorb only a few H+. Food mixed with hydrochloric acid from the stomach would burn the small intestine, the next portion of the digestive tract after the stomach, if it were not for the release of bicarbonate (HCO3–), a weak base that attracts H+. Bicarbonate accepts some of the H+ protons, thereby reducing the acidity of the solution.
What is pH?
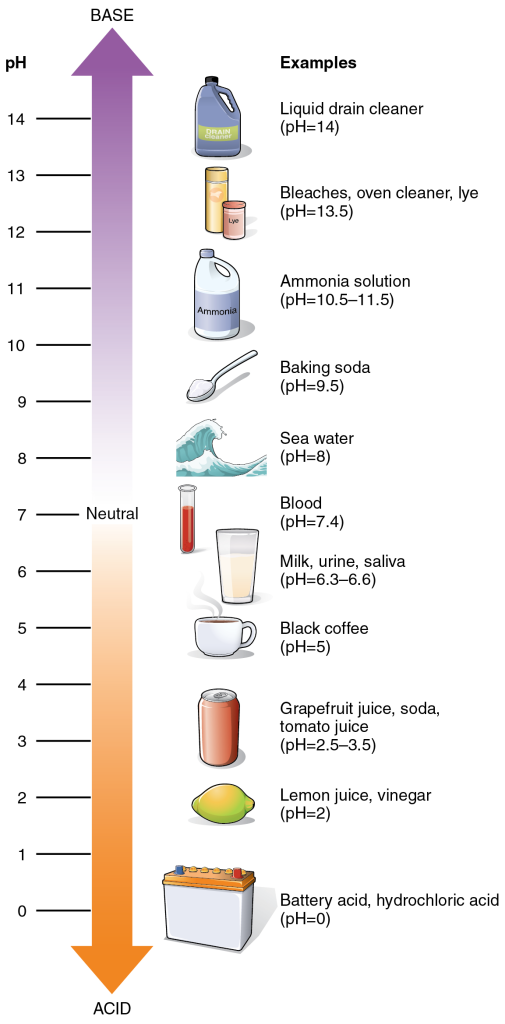
The pH of a solution is a measure of its acidity or its alkalinity (or how basic it is). You have probably used litmus paper, paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator, to test how much acid or base exists in a solution. You might have even used some to make sure the water in an outdoor swimming pool is properly treated. In both cases, this pH test measures the amount of hydrogen ions that exist in a given solution. High concentrations of hydrogen ions yield a low pH, whereas low levels of hydrogen ions result in a high pH. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale (Figure 4.8). Therefore, the more hydrogen ions present, the lower the pH; conversely, the fewer hydrogen ions, the higher the pH.
The pH scale ranges from 0 to 14. A change of one unit on the pH scale represents a change in the concentration of hydrogen ions by a factor of 10, a change in two units represents a change in the concentration of hydrogen ions by a factor of 100. Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. Pure water is neutral. It is neither acidic nor basic and has a pH of 7.0. Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. The blood in your veins is slightly alkaline (pH = 7.4). The environment in your stomach is highly acidic (pH = 1 to 2). Orange juice is mildly acidic (pH = approximately 3.5), whereas baking soda is basic/alkaline (pH = 9.0).
How do buffers work?
Most cells in our bodies operate within a very narrow window of the pH scale, typically ranging only from 7.2 to 7.6. If the pH of the body is outside of this range, the respiratory system malfunctions, as do other organs in the body. Cells no longer function properly, and proteins will break down. Deviation outside of the pH range can induce coma or even cause death.
So how is it that we can ingest or inhale acidic or basic substances and not die? Buffers are the key. Buffers readily absorb excess H+ or OH–, keeping the pH of the body carefully maintained in the aforementioned narrow range. Carbon dioxide is part of a prominent buffer system in the human body; it helps keep the pH within the proper range. This buffer system involves carbonic acid (H2CO3) and bicarbonate (HCO3–) anion. If too much H+ enters the body, bicarbonate will combine with the H+ to create carbonic acid and limit the decrease in pH. Likewise, if too much OH– is introduced into the system, carbonic acid will rapidly dissociate into bicarbonate and H+ ions. The H+ ions can combine with the OH– ions, limiting the increase in pH. While carbonic acid is an important product in this reaction, its presence is fleeting because the carbonic acid is released from the body as carbon dioxide gas each time we breathe. We will look at this reaction in more detail below. Without this buffer system, the pH in our bodies would fluctuate too much and we would fail to survive.
Let us now consider what would happen if the pH of blood did not remain in its normal range. The pH of human blood normally ranges from 7.35 to 7.45, although it is typically identified as pH 7.4. At this slightly basic pH, blood can reduce the acidity resulting from the carbon dioxide (CO2) constantly being released into the bloodstream by the trillions of cells in the body. Homeostatic mechanisms (along with exhaling CO2 while breathing) normally keep the pH of blood within this narrow range. This is critical because fluctuations—either too acidic or too alkaline—can lead to life-threatening disorders as discussed below.
How does the body manage changes in pH?
The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the chemical buffers in the blood to make adjustments to pH. The respiratory tract can adjust the blood pH upward in minutes by exhaling CO2 from the body. The renal system can also adjust blood pH through the excretion of hydrogen ions (H+) and the conservation of bicarbonate, but this process takes hours to days to have an effect. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The kidneys help control acid-base balance by excreting hydrogen ions and generating bicarbonate that helps maintain blood plasma pH within a normal range. Protein buffer systems work predominantly inside cells.
Protein buffers in blood plasma and cells
Nearly all proteins can function as buffers. Proteins are made up of amino acids, which contain positively charged amino groups and negatively charged carboxyl groups. The charged regions of these molecules can bind hydrogen and hydroxyl ions, and thus function as buffers. Buffering by proteins accounts for two-thirds of the buffering power of the blood and most of the buffering within cells.
Bicarbonate-carbonic acid buffer
The bicarbonate-carbonic acid buffer is one of the main buffer systems in the body. pH is regulated through the lungs and the kidneys with this buffer system. When carbon dioxide is produced by cells during cellular respiration, it combines with water to form carbonic acid, which is a weak acid. We have seen that when in water, some of the molecules of a weak acid will dissociate to form H+ ions and another ion, in this case the bicarbonate ion HCO3–
CO2+ H2O → H2CO3 → HCO3– + H+
This reaction will decrease the pH of the blood, or make it more acidic.
This reaction is actually reversible, and we would represent that in an equation with special double arrows ⇌ which show that the reaction can go in either direction.
CO2+ H2O ⇌ H2CO3 ⇌ HCO3– + H+
When a reaction is reversible, it generally reaches a point where the forward reaction and the reverse reaction are happening at the same rate, and we call this dynamic equilibrium. It is dynamic because we can move it, it is not fixed. So, if we were doing a lot of exercise and not getting rid of the carbon dioxide, then the reaction would go forward. If we were getting rid of one or both of the product ions, this would also drive the reaction forward. If, on the other hand, we were getting rid of the carbon dioxide, this would drive the reaction in the reverse direction, resulting in the pH of the blood rising, or becoming more alkaline.
One of the ways the body uses this buffer system to help maintain blood pH is by increasing or decreasing the activity of the respiratory system to manage the amount of carbon dioxide in the blood. The other way is through the urinary system, which controls the level of bicarbonate ions.
Respiratory regulation of acid-base balance
The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (Figure 4.9). CO2 in the blood readily reacts with water to form carbonic acid, and the levels of CO2 and carbonic acid in the blood are in equilibrium. When the CO2 level in the blood rises (as it does when you hold your breath), the excess CO2 reacts with water to form additional carbonic acid, lowering blood pH. Increasing the rate and/or depth of respiration (which you might feel the “urge” to do after holding your breath) allows you to exhale more CO2. The loss of CO2 from the body reduces blood levels of carbonic acid and thereby adjusts the pH upward, toward normal levels. As you might have surmised, this process also works in the opposite direction. Excessive deep and rapid breathing (as in hyperventilation) rids the blood of CO2 and reduces the level of carbonic acid, making the blood too alkaline. This brief alkalosis can be remedied by rebreathing air that has been exhaled into a paper bag. Rebreathing exhaled air will rapidly bring blood pH down toward normal.
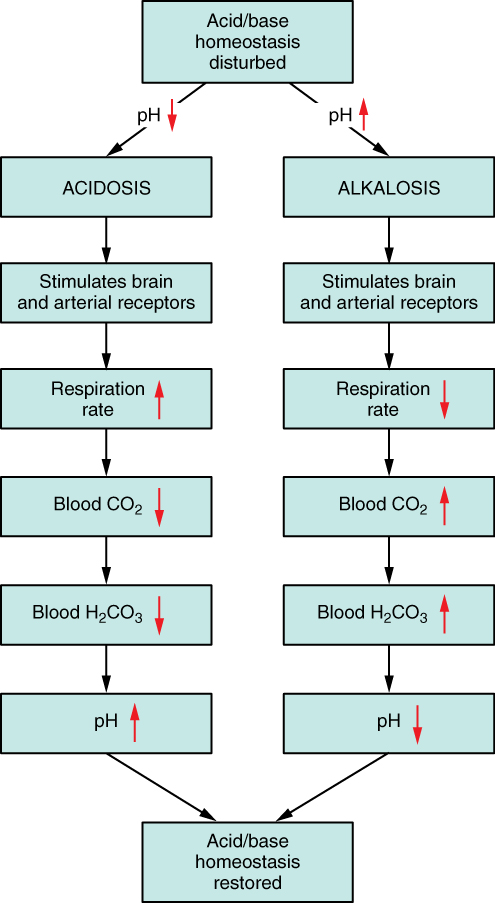
The chemical reactions that regulate the levels of CO2 and carbonic acid occur in the lungs when blood travels through the lung’s pulmonary capillaries. Minor adjustments in breathing are usually sufficient to adjust the pH of the blood by changing how much CO2 is exhaled. In fact, doubling the respiratory rate for less than 1 minute, removing extra CO2, would increase the blood pH by 0.2. This situation is common if you are exercising strenuously over a period of time. To keep up the necessary energy production, you would produce excess CO2 (and lactic acid if exercising beyond your aerobic threshold). In order to balance the increased acid production, the respiration rate goes up to remove the CO2. This helps to keep you from developing acidosis.
The body regulates the respiratory rate by the use of chemoreceptors, which primarily use CO2 as a signal. Peripheral blood sensors are found in the walls of the aorta and carotid arteries. These sensors signal the brain to provide immediate adjustments to the respiratory rate if CO2 levels rise or fall. Yet other sensors are found in the brain itself. Changes in the pH of CSF affect the respiratory centre in the medulla oblongata, which can directly modulate breathing rate to bring the pH back into the normal range. Keep this role in mind when we come to look at the respiratory system in Chapter 9: Beating and breathing.
You may have guessed already that regulation of the body’s acid-base balance can also be changing the blood levels of bicarbonate. This is carried out by the kidneys and will not be covered in the subject.
Acid-Base Balance: Ketoacidosis
Diabetic acidosis, or ketoacidosis, occurs most frequently in people with poorly controlled diabetes mellitus. When certain tissues in the body cannot get adequate amounts of glucose, they depend on the breakdown of fatty acids for energy. When acetyl groups break off the fatty acid chains, the acetyl groups then non-enzymatically combine to form ketone bodies, acetoacetic acid, beta-hydroxybutyric acid, and acetone, all of which increase the acidity of the blood. In this condition, the brain isn’t supplied with enough of its fuel—glucose—to produce all of the ATP it requires to function. Ketoacidosis can be severe and, if not detected and treated properly, can lead to diabetic coma, which can be fatal. A common early symptom of ketoacidosis is deep, rapid breathing as the body attempts to get rid of CO2 and compensate for the acidosis. Another common symptom is fruity-smelling breath, due to the exhalation of acetone. Other symptoms include dry skin and mouth, a flushed face, nausea, vomiting, and stomach pain. Treatment for diabetic coma is ingestion or injection of sugar; its prevention is the proper daily administration of insulin. A person who is diabetic and uses insulin can initiate ketoacidosis if a dose of insulin is missed. Among people with type 2 diabetes, those of Hispanic and African-American descent are more likely to go into ketoacidosis than those of other ethnic backgrounds, although the reason for this is unknown.
acid a substance that releases hydrogen ions (H+) in solution
base a substance that releases hydroxyl ions (0H–) in solution
buffer a solution that can resist pH change upon the addition of an acidic or basic components
colloid a mixture where very small particles of one substance are evenly distributed throughout another substance
electrolytes ions that are capable of conducting an electrical current when in solution
hydrophilic water-loving
hydrophobic water hating
pH a measure of acidity or alkalinity of water-soluble substances
monomer a molecule that may react chemically with another molecule of the same type to form a larger molecule called a polymer
salt a substance that, when dissolved in water, dissociates into ions other than H+ or OH–
solution a mixture of substances where one of the substances dissolves in the other
solute the substance that is dissolved in the solvent
solvent (liquid) the compound of a solution that is present in the greatest amount
suspension a mixture of liquid and solid particles where solid particles will eventually separate and settle if left undisturbed
Review Questions
Chapter attribution
Content adapted from:
Anatomy and Physiology 2e (2022), by J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble and Peter DeSaix, is published by OpenStax https://openstax.org/details/books/anatomy-and-physiology-2e, and used under a CC BY licence.
Concepts of Biology (2013), by Samantha Fowler, Rebecca Roush and James Wise, is published by OpenStax https://openstax.org/details/books/concepts-biology, and used under a CC BY licence.
Microbiology (2016), by Nina Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister and Brian M. Forster, is published by OpenStax https://openstax.org/details/books/microbiology, and used under a CC BY licence.
Media Attributions
- Figure 4.1 drip_drop_of_water_close_nature_green_surface_tension_water_raindrop-992836 © Unknown is licensed under a CC0 (Creative Commons Zero) license
- Figure 4.2 Distribution of fluid in the body © Welcome1To1The1Jungle is licensed under a CC BY (Attribution) license
- Figure 4.3 Hydrogen bonding in water © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix is licensed under a CC BY (Attribution) license
- Figure 4.4 Dehydration synthesis and Hydrolysis reactions © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix is licensed under a CC BY (Attribution) license
- Figure 4.5 Fluid compartments in the human body © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix is licensed under a CC BY (Attribution) license
- Figure 4.6 Dissociation of sodium chloride in water © OpenStax is licensed under a CC BY (Attribution) license
- Figure 4.7 Acids and Bases © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix is licensed under a CC BY (Attribution) license
- Figure 4.8 The pH scale © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix is licensed under a CC BY (Attribution) license
- Figure 4.9 Respiratory regulation of blood pH © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix is licensed under a CC BY (Attribution) license

