8. Essential reactions of life

- Explain the role of enzymes in a cell
- Explain the role of ATP in the cell
- Describe how ATP is generated from glucose metabolism in glycolysis
- Explain how the citric acid cycle and oxidative phosphorylation produce ATP from the products of glycolysis and how carbon dioxide and water are formed in the process
- Explain which pathways of ATP production are available in the presence and absence of oxygen.
8.1 | What is metabolism?
One characteristic of a living organism is metabolism, which is the sum total of all of the chemical reactions that go on to maintain that organism’s health and life. The bonding processes you have learned thus far are anabolic chemical reactions; that is, they form larger molecules from smaller molecules or atoms. However, recall that metabolism can proceed in another direction: in catabolic chemical reactions, bonds between components of larger molecules break, releasing smaller molecules or atoms. Both types of reaction involve exchanges not only of matter, but of energy.
What is the role of energy in the chemical reactions of metabolism?
Chemical reactions require a sufficient amount of energy to cause the matter to collide with enough precision and force that old chemical bonds can be broken, and new ones formed. In general, kinetic energy is the form of energy powering any type of matter in motion. Imagine you are building a brick wall. The energy it takes to lift and place one brick atop another is kinetic energy—the energy matter possesses because of its motion. Once the wall is in place, it stores potential energy. Potential energy is the energy of position, or the energy matter possesses because of the positioning or structure of its components. If the brick wall collapses, the stored potential energy is released as kinetic energy as the bricks fall.
In the human body, potential energy is stored in the bonds between atoms and molecules. Chemical energy is the form of potential energy in which energy is stored in chemical bonds. When those bonds are formed, chemical energy is invested, and when they break, chemical energy is released. Notice that chemical energy, like all energy, is neither created nor destroyed; rather, it is converted from one form to another. When you eat an energy bar before heading out the door for a hike, the honey, nuts, and other foods the bar contains are broken down and rearranged by your body into molecules that your muscle cells convert to kinetic energy.
What are chemical reactions again?
We have covered the basics of chemical reactions in Chapter 3: Chemistry for life. Recall that, all chemical reactions begin with a reactant, the general term for the one or more substances that enter into the reaction. The one or more substances produced by a chemical reaction are called the product.
We also touched on the direction of a chemical reaction in Chapter 3: Chemistry for life and Chapter 4: Water biology where we saw that chemical reactions can proceed in either direction under the right conditions. For example, reactants may synthesise into a product that is later decomposed. This reversibility of a chemical reaction is indicated with a double arrow: A + BC ⇄ AB + C. We saw that this was the case in maintaining blood pH, where the buffer reaction could be “pushed” one way or the other.
How can we make chemical reactions go fast enough for life?
If you pour vinegar into baking soda, the reaction is instantaneous; the concoction will bubble and fizz. But many chemical reactions take time. A variety of factors influence the rate of chemical reactions. This section, however, will consider only those most important in human functioning.
Properties of the reactants
If chemical reactions are to occur quickly, the atoms in the reactants have to have easy access to one another. Thus, the greater the surface area of the reactants, the more readily they will interact. When you pop a cube of cheese into your mouth, you chew it before you swallow it. Among other things, chewing increases the surface area of the food so that digestive chemicals can more easily get at it. As a general rule, gases tend to react faster than liquids or solids, again because it takes energy to separate particles of a substance, and gases by definition already have space between their particles. Similarly, the larger the molecule, the greater the number of total bonds, so reactions involving smaller molecules, with fewer total bonds, would be expected to proceed faster.
In addition, recall that some elements are more reactive than others. Reactions that involve highly reactive elements like hydrogen proceed more quickly than reactions that involve less reactive elements. Reactions involving stable elements like helium are not likely to happen at all.
Temperature
Nearly all chemical reactions occur at a faster rate at higher temperatures. Recall that kinetic energy is the energy of matter in motion. The kinetic energy of subatomic particles increases in response to increases in thermal energy. The higher the temperature, the faster the particles move, and the more likely they are to come in contact and react.
Concentration and pressure
If just a few people are dancing at a club, they are unlikely to step on each other’s toes. But as more and more people get up to dance—especially if the music is fast—collisions are likely to occur. It is the same with chemical reactions: the more particles present within a given space, the more likely those particles are to bump into one another. This means that chemists can speed up chemical reactions not only by increasing the concentration of particles—the number of particles in the space—but also by decreasing the volume of the space, which would correspondingly increase the pressure. If there were 100 dancers in that club, and the manager abruptly moved the party to a room half the size, the concentration of the dancers would double in the new space, and the likelihood of collisions would increase accordingly.
Enzymes and other catalysts
For two chemicals in nature to react with each other they first have to come into contact, and this occurs through random collisions. Because heat helps increase the kinetic energy of atoms, ions, and molecules, it promotes their collision. But in the body, extremely high heat—such as a very high fever—can damage body cells and be life-threatening. On the other hand, normal body temperature is not high enough to promote the chemical reactions that sustain life. That is where catalysts come in.
In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any change. You can think of a catalyst as a chemical change agent. Catalysts help increase the rate and force at which atoms, ions, and molecules collide, thereby increasing the probability that their valence shell electrons will interact.
The most important catalysts in the human body are enzymes. An enzyme is a catalyst primarily composed of protein; enzymes work by lowering the level of energy that needs to be invested in a chemical reaction. A chemical reaction’s activation energy is the threshold level of energy needed to break the bonds in the reactants. Once those bonds are broken, new arrangements can form. Without an enzyme to act as a catalyst, a much larger investment of energy is needed to ignite a chemical reaction (Figure 8.2).
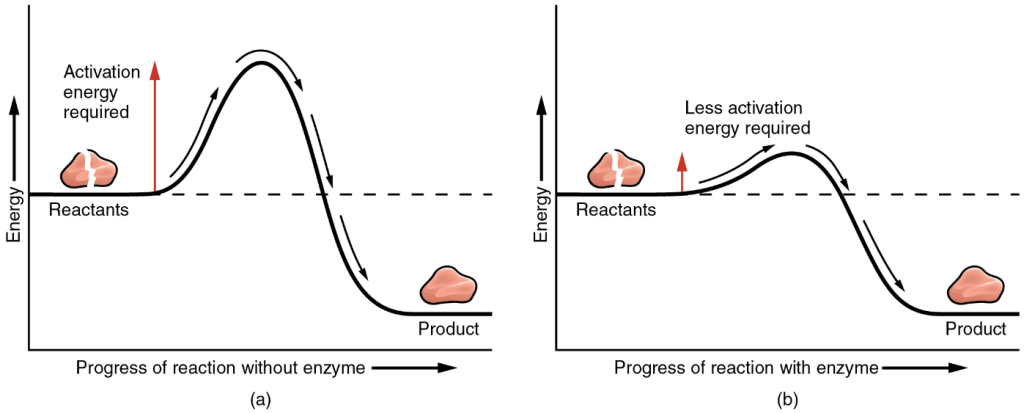
Enzymes are critical to the body’s healthy functioning. They assist, for example, with the breakdown of food and its conversion to energy. In fact, most of the chemical reactions in the body are facilitated by enzymes. Without them, studies have estimated that certain biological processes and some essential reactions would take a trillion years to occur, whereas the participation of specific enzymes could accomplish these same tasks in one hundredth of a second! I think we all are a little too impatient to wait for reactions to happen on their own, so let us discuss how enzymes perform this important duty within our cells.
Each enzyme is specialised to its own particular task; for instance, sucrase is only capable of breaking down sucrose sugars, and DNA ligase is responsible for bonding DNA nucleotides together. We have seen that proteins have very specific three dimensional structures, and enzymes are proteins. Enzymes interact with their substrate at an active site that is specific for the substrate. For many years, scientists through that enzyme-substrate binding took place in a simple lock-and-key fashion. This model asserted that the enzyme and substrate fit together perfectly in one instantaneous step, like a key fitting into a lock. However, current research supports a more refined view called induced fit (Figure 8.3). This model expands on the lock-and-key model by describing a more dynamic interaction between enzyme and substrate. As the enzyme and substrate come together, interactions between the molecules cause a slight conformational change in the enzyme’s structure that allows the enzyme and substrate to fit together strongly and carry out the reaction.
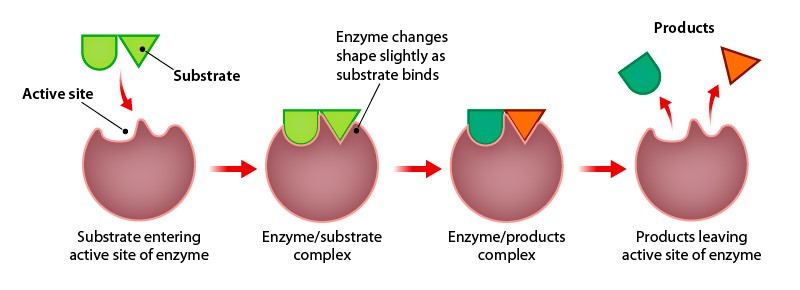
Environmental conditions such as pH and temperature can affect the rate at which enzymes function due to modifying enzyme-substrate interaction through shape change or molecular movement. Importantly, enzymes are not consumed in the reaction and can be reused many times over.
8.2 | What is cellular energy and how do cells make it?
Virtually every task performed by living organisms requires energy. Energy is needed to perform heavy labour and exercise, but humans also use energy while thinking, and even during sleep. In fact, the living cells of every organism constantly use energy. Nutrients and other molecules are imported into the cell, metabolised (broken down) and possibly synthesised into new molecules, modified if needed, transported around the cell, and possibly distributed to the entire organism. For example, the large proteins that make up muscles are built from smaller molecules imported from dietary amino acids. Ingested complex carbohydrates are broken down into simple sugars that the cell uses for energy. Just as energy is required to both build and demolish a building, energy is required for the synthesis and breakdown of molecules as well as the transport of molecules into and out of cells. In addition, processes such as ingesting and breaking down pathogenic bacteria and viruses, exporting wastes and toxins, and movement of the cell require energy. From where, and in what form, does this energy come? How do living cells obtain energy, and how do they use it? At the end of this chapter, you will be able to name the metabolic pathways cells use to harness chemical energy from the breakdown of carbohydrates and fat and describe how these pathways allow cells to produce ATP to power cellular processes.
Metabolic pathways
Consider the metabolism of sugar. This is a classic example of one of the many cellular processes that use and produce energy. Living things consume sugars such as monosaccharides like glucose and fructose, or disaccharides like sucrose as a major energy source because sugar molecules have a great deal of energy stored within their bonds. Energy-storage molecules such as glucose are consumed only to be broken down to use their energy. However, the energy from the breakdown of the sugars is stored in the form of ATP molecules before it can be used for cellular processes. Just as the dollar is used as currency to buy goods, cells use molecules of ATP as energy currency to perform immediate work.
The process for breaking down sugar molecules to produce ATP actually involves three metabolic pathways. A metabolic pathway is a series of chemical reactions that takes a starting molecule and modifies it, step-by-step, through a series of metabolic intermediates, eventually yielding a final product.
It is important to remember that the chemical reactions of metabolic pathways do not take place on their own. Each reaction step is facilitated, or catalysed, by an enzyme. Enzymes are important for catalysing all types of biological reactions—those that require energy as well as those that release energy.
What is adenosine triphosphate (ATP)?
Within the cell, where does energy to power the essential chemical reactions come from? The answer lies with an energy-supplying molecule called adenosine triphosphate, or ATP. ATP is a small, relatively simple molecule, but within its bonds contains the potential for a quick burst of energy that can be harnessed to perform cellular work. Recall that this molecule can be thought of as the primary energy currency of cells in the same way that money is the currency that people exchange for things they need. ATP is used to power the majority of energy-requiring cellular reactions.
ATP in living systems
A living cell cannot store significant amounts of free energy. Excess free energy would result in an increase of heat in the cell, which would denature enzymes and other proteins, and thus destroy the cell. Rather, a cell must be able to store energy safely and release it for use only as needed. Living cells accomplish this using ATP, which can be used to fill any energy need of the cell. How? It functions as a rechargeable battery.
When ATP is broken down, usually by the removal of its terminal phosphate group, energy is released. This energy is used by the cell to do work, usually by the binding of the released phosphate to another molecule, thus activating it; for example, in the mechanical work of muscle contraction, ATP supplies energy to move the contractile muscle proteins.
ATP structure and function
At the heart of ATP is a molecule of adenosine monophosphate (AMP), which is composed of an adenine molecule bonded to both a ribose molecule and a single phosphate group (Figure 8.4). Recall that ribose is the five-carbon sugar found in RNA and that adenine is one of the nucleotides in RNA (refer to Chapter 6: Coding life). The addition of a second phosphate group to this core molecule results in adenosine diphosphate (ADP); the addition of a third phosphate group forms adenosine triphosphate (ATP).
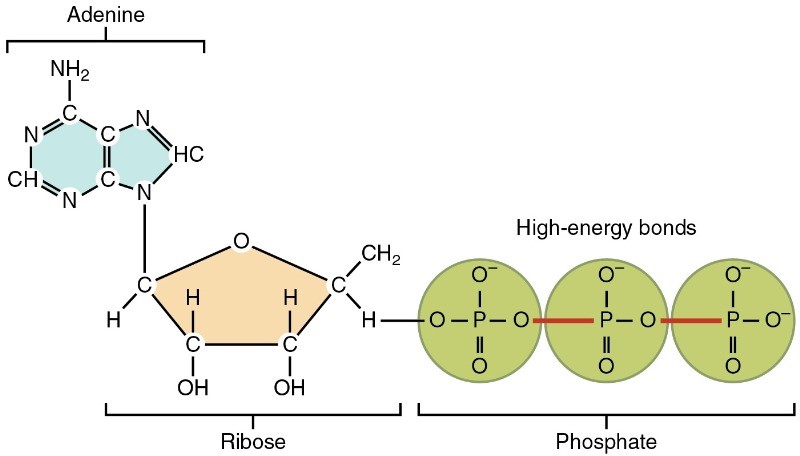
The addition of a phosphate group to a molecule requires a high amount of energy and results in a high-energy bond. Phosphate groups are negatively charged and thus repel one another when they are arranged in series, as they are in ADP and ATP. This repulsion makes the ADP and ATP molecules inherently unstable. The release of one or two phosphate groups from ATP, a process called hydrolysis, releases energy.
8.3 | What is the process of converting glucose to cellular energy?
There are three key pathways involved in converting the energy in glucose to ATP. These pathways are:
- Glycolysis: takes place in the cytoplasm
- The TCA (tricarboxylic) cycle also known as the Citric Acid or Krebs cycle: takes place in the mitochondria
- Oxidative phosphorylation: takes place in the mitochondria
Glycolysis
Nearly all of the energy used by living things comes to them in the bonds of the sugar, glucose. Glycolysis is the first step in the breakdown of glucose to extract energy for cell metabolism (remember that the term lysis means breakdown). Many living organisms carry out glycolysis as part of their metabolism. Glycolysis takes place in the cytoplasm of most prokaryotic and all eukaryotic cells.
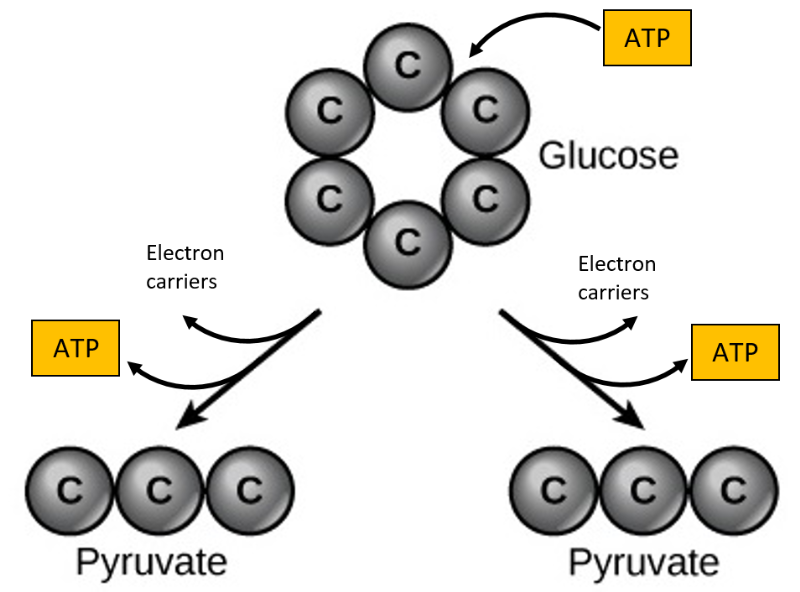
Glycolysis begins with the six-carbon, ring-shaped structure of a single glucose molecule and ends with two molecules of a three-carbon sugar called pyruvate. Glycolysis consists of two distinct phases. In the first part of the glycolysis pathway, 2 molecules of ATP are used. In the second part of glycolysis, 4 molecules of ATP and an electron carrier are produced (Figure 8.5). This means that this process produces 2 molecules of ATP overall.
If the cell cannot catabolise the pyruvate molecules further, it will harvest only two ATP molecules from one molecule of glucose. For example, mature mammalian red blood cells are only capable of glycolysis, which is their sole source of ATP. If glycolysis is interrupted, these cells would eventually die.
Tricarboxylic acid cycle (TCA cycle)
Also called the Krebs cycle and citric acid cycle, the TCA cycle is the second stage of cellular respiration. In eukaryotic cells, the pyruvate molecules produced at the end of glycolysis are transported into mitochondria, which are organelles known as the powerhouses of the cell (refer to Chapter 2: Maintaining a balance).
If oxygen is available, aerobic respiration will go forward. In mitochondria, pyruvate will be transformed into acetyl CoA (Figure 8.6), releasing carbon dioxide.
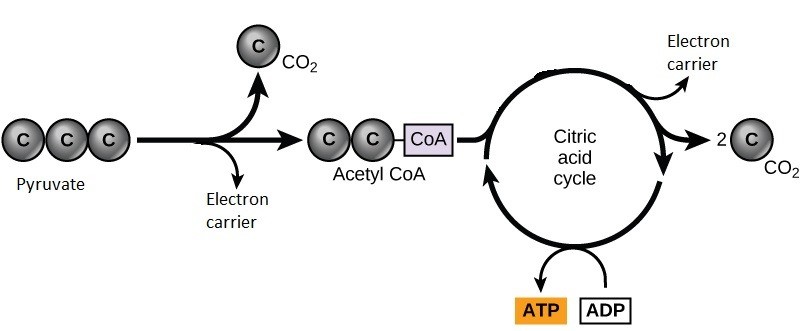
Unlike glycolysis, the TCA cycle is a closed loop: the last part of the pathway regenerates the compound used in the first step. The TCA cycle produces two carbon dioxide molecules, one ATP molecule, and electron carriers (Figure 8.7). Part of this is considered an aerobic pathway (oxygen-requiring) because the electron carriers produced must transfer their electrons to the next pathway in the system, which will use oxygen (oxidative phosphorylation discussed below). If oxygen is not present, this transfer does not occur.
Two carbon atoms come into the citric acid cycle from each acetyl group. Two carbon dioxide molecules are released on each turn of the cycle. After two turns of the cycle, all six carbon atoms from the original glucose molecule will be eventually released as carbon dioxide.
Oxidative phosphorylation
You have just read about two pathways in glucose catabolism—glycolysis and the TCA cycle—that generate ATP. Most of the ATP generated during the aerobic catabolism of glucose, however, is not generated directly from these two pathways. Rather, it occurs in the oxidative phosphorylation stage of cellular respiration when the energy of electron carriers is harnessed to form ATP. Oxidative phosphorylation, which is the last component of aerobic respiration, takes place in the mitochondria and is the only part of metabolism that uses atmospheric oxygen. In animals, oxygen enters the body through the respiratory system for this purpose. Oxidative phosphorylation occurs in two parts:
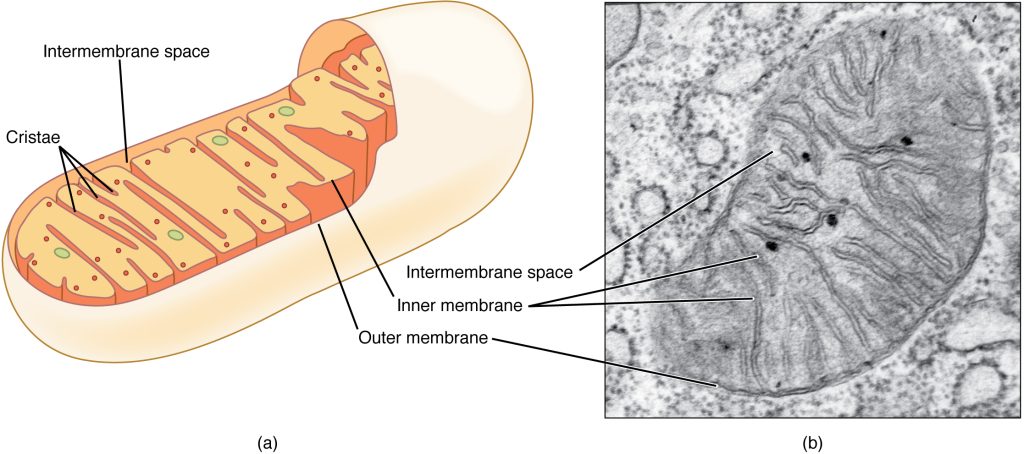
Step 1. The Electron Transport Chain: The electron carriers enter an electron transport chain where their energy is used to pump protons (H+) into the intermembrane space of mitochondria (Figure 8.7); this establishes a proton gradient between the intermembrane space and matrix of the mitochondria. At the end of the electron transport chain, the electrons are transferred to an oxygen atom to produce an oxygen ion (O2-). The negative charge attracts H+ and water is formed (H2O). If there were no oxygen present in the mitochondrion, the electrons could not be removed from the system, and the entire electron transport chain would back up and stop. The mitochondria would be unable to generate new ATP in this way, and the cell would ultimately die from lack of energy. This is the reason we must breathe to draw in new oxygen.
Step 2. Chemiosmosis: The flow of protons down their concentration gradient from the intermembrane space, across the membrane through the membrane protein called ATP synthase, into the matrix of the mitochondria leads to the formation of ATP.
Chemiosmosis is used to generate 90 percent of the ATP made during aerobic glucose catabolism. The result of the reactions is the production of ATP from the energy of the electrons removed from hydrogen atoms. These atoms were originally part of a glucose molecule.
Animation of chemiosmosis [3:48]
The electron transport chain and the production of ATP through chemiosmosis are collectively called oxidative phosphorylation. Watch this YouTube explanation of oxidative phosphorylation.
ATP Yield
Overall, in living systems, these pathways of glucose catabolism extract about 34 percent of the energy contained in glucose.
Career Connection: Mitochondrial Disease Physician
What happens when the critical reactions of cellular respiration do not proceed correctly? Mitochondrial diseases are genetic disorders of metabolism. Mitochondrial disorders can arise from mutations in nuclear or mitochondrial DNA, and they result in the production of less energy than is normal in body cells. Symptoms of mitochondrial diseases can include muscle weakness, lack of coordination, stroke-like episodes, and loss of vision and hearing. Most affected people are diagnosed in childhood, although there are some adult-onset diseases. Identifying and treating mitochondrial disorders is a specialised medical field. The educational preparation for this profession requires a medical degree followed by a specialisation in medical genetics. Medical geneticists can be board-certified by the American Board of Medical Genetics and go on to become associated with professional organisations devoted to the study of mitochondrial disease, such as the Mitochondrial Medicine Society and the Society for Inherited Metabolic Disease.
8.4 | What happens if there is not enough oxygen available?
In aerobic respiration, the final electron acceptor is an oxygen molecule, O2. If aerobic respiration occurs, then ATP will be produced using the energy of the high-energy electrons carried by the electron carriers to the electron transport chain. If aerobic respiration does not occur, the electron carriers must get rid of their electrons another way so that the electron carrier can be reused for glycolysis to continue. This process is called anaerobic cellular respiration. Anaerobic respiration enables organisms to convert energy for their use in the absence of oxygen.
Lactic acid (or lactate) fermentation
The anaerobic respiration method used by animals and some bacteria like those in yogurt is lactate fermentation (Figure 8.10). This occurs routinely in mammalian red blood cells and in skeletal muscle that has insufficient oxygen supply to allow aerobic respiration to continue (that is, in muscles used to the point of fatigue). In muscles, lactic acid produced by fermentation must be removed by the blood circulation and brought to the liver for further metabolism.
8.5 | What about the fats and proteins that we eat?
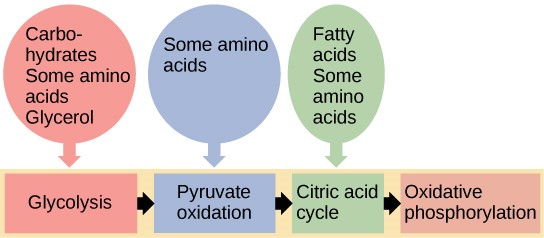
You have learned about the catabolism of glucose, which provides energy to living cells. But living things consume more than just glucose for food. How does a turkey sandwich, which contains protein, provide energy to your cells? This happens because all of the catabolic pathways for carbohydrates, proteins, and lipids eventually connect into glycolysis and the citric acid cycle pathways (Figure 8.8).
Connections of other sugars to glucose metabolism
Glycogen, a polymer of glucose, is a short-term energy storage molecule in animals. When there is adequate ATP present, excess glucose is converted into glycogen for storage. Glycogen is made and stored in the liver and muscle. Glycogen will be taken out of storage if blood sugar levels drop. The presence of glycogen in muscle cells as a source of glucose allows ATP to be produced for a longer time during exercise.
Sucrose is a disaccharide made from glucose and fructose bonded together. Sucrose is broken down in the small intestine, and the glucose and fructose are absorbed separately. Fructose is one of the three dietary monosaccharides, along with glucose and galactose (which is part of milk sugar, the disaccharide lactose), that are absorbed directly into the bloodstream during digestion. The catabolism of both fructose and galactose produces the same number of ATP molecules as glucose.
Connections of proteins to glucose metabolism
Proteins are broken down by a variety of enzymes in cells. Most of the time, amino acids are recycled into new proteins. If there are excess amino acids, however, or if the body is in a state of famine, some amino acids will be shunted into glycolysis and the citric acid cycle. Each amino acid must have its amino group removed prior to entry into these pathways. The amino group is converted into ammonia. In mammals, the liver synthesises urea from two ammonia molecules and a carbon dioxide molecule. Thus, urea is the principal waste product in mammals from the nitrogen originating in amino acids, and it leaves the body in urine.
Connections of lipids to glucose metabolism
The lipids that are connected to the glucose pathways are cholesterol and triglycerides. Cholesterol is a lipid that contributes to cell membrane flexibility and is a precursor of steroid hormones. The synthesis of cholesterol starts with acetyl CoA and proceeds in only one direction. The process cannot be reversed, and ATP is not produced.
Triglycerides are a form of long-term energy storage in animals. Triglycerides store about twice as much energy as carbohydrates. Triglycerides are made of glycerol and three fatty acids. Animals can make most of the fatty acids they need. Triglycerides can be both made and broken down through parts of the glucose catabolism pathways. Glycerol is metabolised in glycolysis. Fatty acids are converted to acetyl CoA and enter the citric acid cycle.
Key Terms
acetyl CoA the combination of an acetyl group derived from pyruvic acid and coenzyme A which is made from pantothenic acid (a B-group vitamin)
activation energy the amount of initial energy necessary for reactions to occur
active site a specific region on the enzyme where the substrate binds
aerobic respiration the process of producing energy where oxygen is the final electron acceptor
anabolic describes the pathway that requires a net energy input to synthesise complex molecules from simpler ones
anaerobic cellular respiration the use of an electron acceptor other than oxygen to produce energy
ATP or adenosine triphosphate the cell’s energy currency
ATP synthase a membrane-embedded protein complex that regenerates ATP from ADP with energy from protons diffusing through it
bioenergetics the concept of energy flow through living systems
catabolic describes the pathway that releases net energy when complex molecules are broken down into simpler ones
catalyst a substance that increases the rate of a chemical reaction without itself undergoing any change
cellular respiration the process of extracting energy in the form of ATP from glucose
chemical energy the form of potential energy in which energy is stored in chemical bonds
chemical gradient the difference in concentration of a solute across a membrane or space
chemiosmosis the movement of hydrogen ions down their electrochemical gradient across a membrane through ATP synthase to generate ATP
citric acid cycle a series of enzyme-catalysed chemical reactions of central importance in all living cells that harvests the energy in carbon-carbon bonds of sugar molecules to generate ATP; the citric acid cycle is an aerobic metabolic pathway because it requires oxygen in later reactions to proceed
decomposition reaction a chemical reaction that breaks down or “de-composes” something larger into its constituent part
electron transport chain a series of four large, multi-protein complexes embedded in the inner mitochondrial membrane that accepts electrons from donor compounds and harvests energy from a series of chemical reactions to generate a hydrogen ion gradient across the membrane
enzyme a catalyst primarily composed of protein
fermentation the steps that follow the partial oxidation of glucose via glycolysis to regenerate NAD+; occurs in the absence of oxygen and uses an organic compound as the final electron acceptor
glycolysis the process of breaking glucose into two three-carbon molecules with the production of ATP and NADH
kinetic energy is the form of energy powering any type of matter in motion
metabolism all the chemical reactions that take place inside cells, including those that use energy and those that release energy
oxidative phosphorylation the production of ATP by the transfer of electrons down the electron transport chain to create a proton gradient that is used by ATP synthase to add phosphate groups to ADP molecules
potential energy the type of energy that refers to the potential to do work
product the one or more substances produced by a chemical reaction
reactant substance that enter into a chemical reaction
substrate a molecule on which the enzyme acts
synthesis reaction a chemical reaction that results in the synthesis (joining) of components that were formerly separate
Review Questions
Chapter attribution
Content adapted from:
Anatomy and Physiology 2e (2022), by J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble and Peter DeSaix, is published by OpenStax https://openstax.org/details/books/anatomy-and-physiology-2e, and used under a CC BY licence.
Concepts of Biology (2013), by Samantha Fowler, Rebecca Roush and James Wise, is published by OpenStax https://openstax.org/details/books/concepts-biology, and used under a CC BY licence.
Microbiology (2016), by Nina Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister and Brian M. Forster, is published by OpenStax https://openstax.org/details/books/microbiology, and used under a CC BY licence.
Media Attributions
- Figure 8.1 High jumper © tableatny is licensed under a CC BY (Attribution) license
- Figure 8.2 Activation energy © Authors: J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix is licensed under a CC BY (Attribution) license
- Figure 8.3 Lock and key model of enzyme function © Tim Vickers is licensed under a Public Domain license
- Figure 8.4 Molecular structure of ATP © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix adapted by Vicki Dunk is licensed under a CC BY (Attribution) license
- Figure 8.5 Overview of Glycolysis © Samantha Fowler, Rebecca Roush & James Wise adapted by Vicki Dunk is licensed under a CC BY (Attribution) license
- Figure 8.6 The link reaction and the citric acid cycle © Samantha Fowler, Rebecca Roush & James Wise adapted by Vicki Dunk is licensed under a CC BY (Attribution) license
- Figure 8.7 Mitochondria © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble &Peter DeSaix is licensed under a CC BY (Attribution) license
- Figure 8.8 © Samantha Fowler, Rebecca Roush, James Wise is licensed under a CC BY (Attribution) license

