3. Chemistry for life
What will I be able to do at the end of this chapter?
- Describe the fundamental composition of matter
- Identify the three subatomic particles
- Explain the relationship between an atom’s number of electrons and its relative stability
- Distinguish between ionic bonds, covalent bonds, and hydrogen bonds
- Explain what a chemical reaction is.
The smallest, most fundamental material components of the human body are basic chemical elements. The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus are the key building blocks of the chemicals found in living things. They form the carbohydrates, nucleic acids, proteins, lipids and water that are the fundamental molecular components of all organisms. The properties of these molecules (called biomolecules or macromolecules) are determined by how their constituent elements interact with each other.
This chapter begins by examining elements by first exploring the structure of the basic unit (atom) of an element. It goes on to discuss how the structure of the atom determines the characteristics of an element. The chapter then explores the concepts behind chemical bonding between elements as this bonding is necessary for the formation of the biomolecules necessary for life.
3.1 | Elements and atoms: The building blocks of matter
The substance of the universe—from a grain of sand to a star—is called matter. Scientists define matter as anything that occupies space and has mass. An object’s mass and its weight are related concepts, but not quite the same. An object’s mass is the amount of matter contained in the object, and the object’s mass is the same whether that object is on Earth or in the zero-gravity environment of outer space. An object’s weight, on the other hand, is its mass as affected by the pull of gravity. Where gravity strongly pulls on an object’s mass, its weight is greater than it is where gravity is less strong. An object of a certain mass weighs less on the moon, for example, than it does on Earth because the gravity of the moon is less than that of Earth. In other words, weight is variable, and is influenced by gravity. A piece of cheese that weighs a kilogram on Earth weighs only a few grams on the moon.
What is the difference between elements and compounds?
All matter in the natural world is composed of one or more of the 92 fundamental substances called elements. An element is a pure substance that is distinguished from all other matter by the fact that it cannot be created or broken down by ordinary chemical means. While your body can assemble many of the chemical compounds needed for life from their constituent elements, it cannot make elements. Each element’s name can be replaced by a one or two-letter symbol; you will become familiar with some of these during this course.
All the elements in your body are derived from the foods you eat and the air you breathe. They must come from the environment. A familiar example of an element that your body requires is calcium (Ca). Calcium is essential to the human body; it is absorbed and used for a number of processes, including strengthening bones. When you consume dairy products your digestive system breaks down the food into components small enough to cross into the bloodstream. Among these is calcium, which, because it is an element, cannot be broken down further. The elemental calcium in cheese, therefore, is the same as the calcium that forms your bones. Some other elements you might be familiar with are oxygen, sodium, and iron. The elements in the human body are shown in Figure 3.2, beginning with the most abundant: oxygen (O), carbon (C), hydrogen (H), and nitrogen (N).
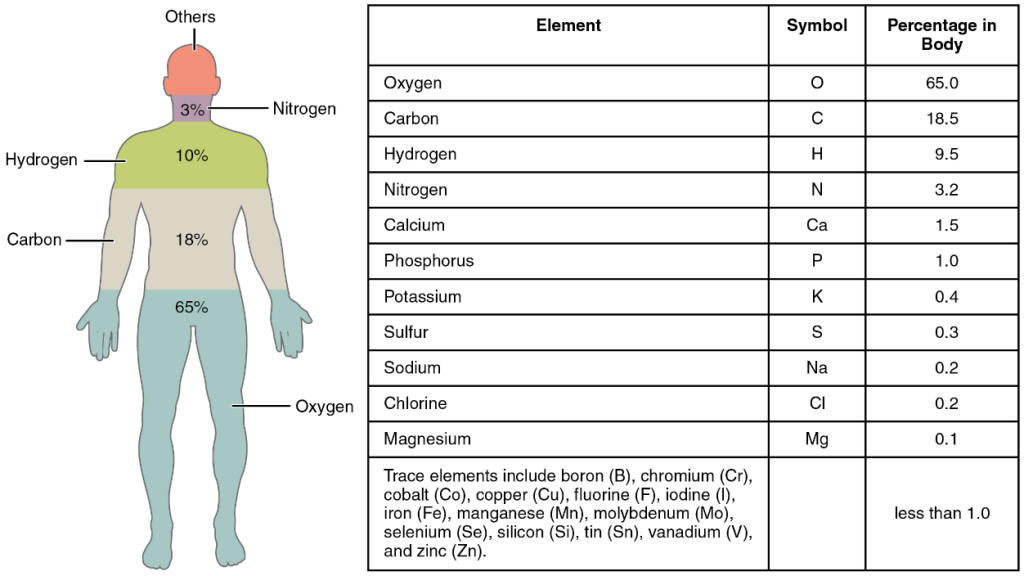
In nature, elements rarely occur alone. Instead, they combine to form compounds. A compound is a substance composed of two or more elements joined by chemical bonds. For example, the compound glucose is an important body fuel. It is always composed of the same three elements: carbon, hydrogen, and oxygen. Moreover, the elements that make up any given compound always occur in the same relative amounts. In glucose, there are always six carbon and six oxygen units for every twelve hydrogen units. But what, exactly, are these “units”?
What are atoms?
In this subject, we will focus only on the first 20 elements, which includes all of the elements in Figure 3.2. In addition, we will be using the simpler planetary model of atomic structure as shown in Figure 3.3, which has the electrons in shells around the nucleus, like planets around the sun.
An atom is the smallest quantity (“unit”) of an element that retains the unique properties of that element. In other words, an atom of hydrogen is a unit of hydrogen—the smallest amount of hydrogen that can exist. As you might guess, atoms are almost unfathomably small; to get an idea of how small, the full stop at the end of this sentence is millions of atoms wide.
Atoms are made up of even smaller subatomic particles: the main ones are:
- the proton
- the neutron
- the electron.
All atoms contain protons, electrons, and neutrons (Figure 3.3 shows a model of the helium atom). The only exception is hydrogen (H), which is made of one proton and one electron. A proton is a positively charged particle that resides in the nucleus (the core of the atom) of an atom and has a mass of 1 and a charge of +1. An electron is a negatively charged particle that travels in the space around the nucleus in an orbit. In other words, it resides outside of the nucleus. It has a negligible mass and has a charge of –1.
Neutrons, like protons, reside in the nucleus of an atom. They have a mass of 1 and no charge. The positive (protons) and negative (electrons) charges balance each other in a neutral atom, which has a net zero charge.

As stated earlier, each element has its own unique properties. Each contains a different number of protons and neutrons, giving it its own atomic number and mass number. The atomic number of an element is equal to the number of protons that element contains. The atomic mass is the number of protons plus the number of neutrons of that element. Therefore, it is possible to determine the number of neutrons by subtracting the atomic number from the mass number. For example, sodium (Na) has a mass number of 23 and atomic number of 11, which means it has 12 neutrons.
Atomic mass = number protons (atomic number) + number of neutrons
These numbers provide information about the elements and how they will react when combined. Different elements have different melting and boiling points, and are in different states (liquid, solid, or gas) at room temperature. They also combine in different ways. Some form specific types of bonds, whereas others do not. How they combine is based on the number of electrons present. Because of these characteristics, the elements are arranged into the periodic table of elements (Figure 3.4), a chart of the elements that includes the atomic number and relative atomic mass of each element. The periodic table also provides key information about the properties of elements—often indicated by colour-coding. The arrangement of the table also shows how the electrons in each element are organised and provides important details about how atoms will react with each other to form molecules.
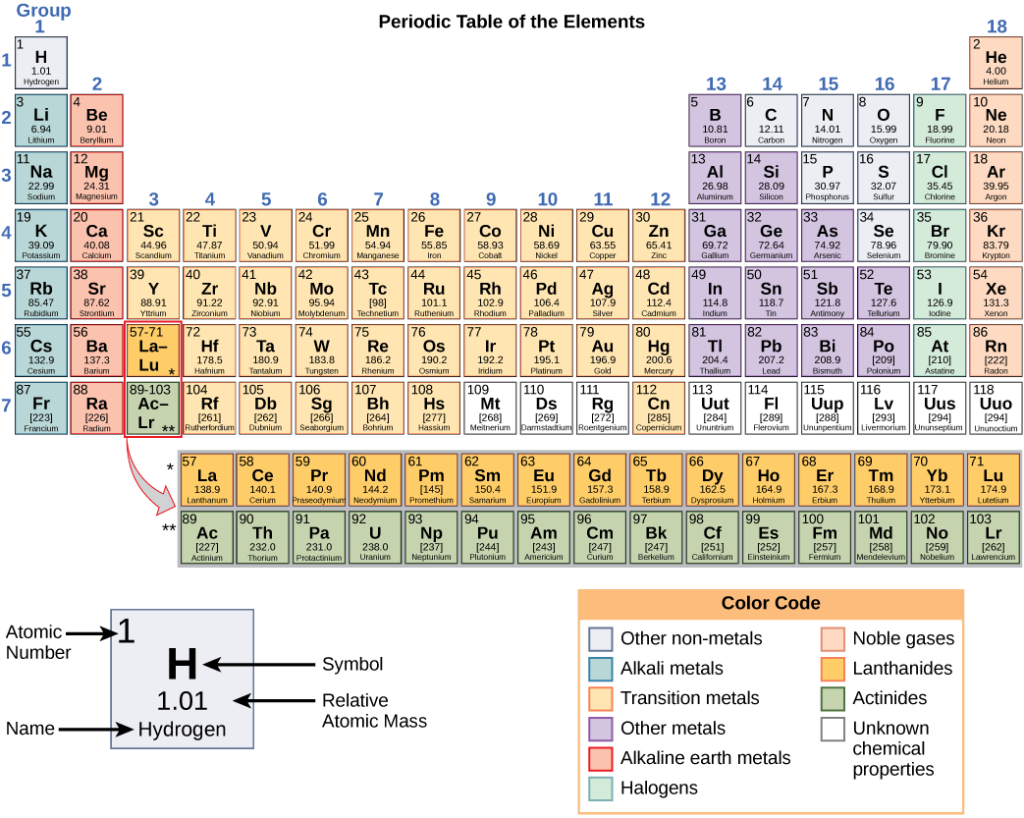
Visit this website to view an interactive periodic table. In the periodic table of the elements, elements in a single column have the same number of electrons that can participate in a chemical reaction. These electrons are known as valence electrons (the concept of valence electrons is discussed later in the chapter). For example, the elements in the first column all have a single valence electron, an electron that can be donated in a chemical reaction with another atom.
What are isotopes?
Isotopes are different forms of the same element that have the same number of protons, but a different number of neutrons. Some elements, such as carbon, potassium, and uranium, have naturally occurring isotopes. Carbon-12, the most common isotope of carbon, contains six protons and six neutrons. Therefore, it has a mass number of 12 (six protons added to six neutrons) and an atomic number of 6 (which makes it carbon). Carbon-14 contains six protons and eight neutrons. Therefore, it has a mass number of 14 (six protons added to eight neutrons) and an atomic number of 6, meaning it is still the element carbon. These two alternate forms of carbon are isotopes.
Hydrogen has three common isotopes, shown in Figure 3.5
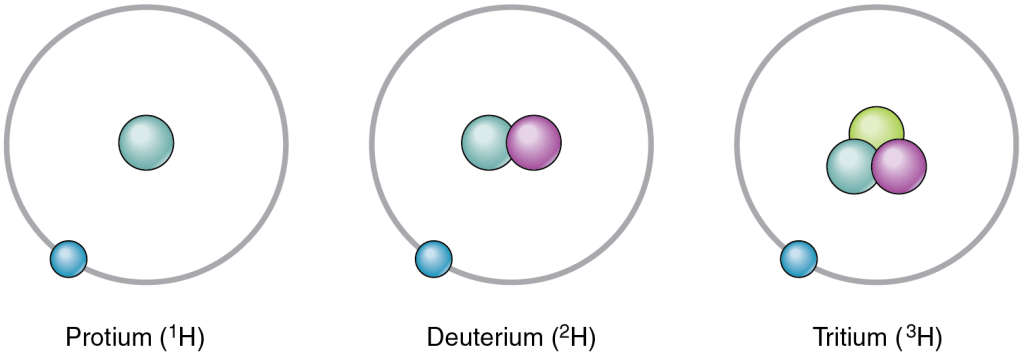
An isotope that contains more than the usual number of neutrons is referred to as a heavy isotope. An example is 14C. Heavy isotopes tend to be unstable, and unstable isotopes are radioactive. A radioactive isotope is an isotope whose nucleus readily decays, giving off subatomic particles and electromagnetic energy. Different radioactive isotopes (also called radioisotopes) differ in their half-life, the time it takes for half of any size sample of an isotope to decay. For example, the half-life of tritium—a radioisotope of hydrogen—is about 12 years, indicating it takes 12 years for half of the tritium nuclei in a sample to decay. Excessive exposure to radioactive isotopes can damage human cells and even cause cancer and birth defects, but when exposure is controlled, some radioactive isotopes can be useful in medicine. For more information, see the Career Connections.
Career connection: Interventional radiologist
The controlled use of radioisotopes has advanced medical diagnosis and treatment of disease. Interventional radiologists are physicians who treat disease by using minimally invasive techniques involving radiation. Many conditions that could once only be treated with a lengthy and traumatic operation can now be treated non-surgically, reducing the cost, pain, length of hospital stay, and recovery time for patients. For example, in the past, the only options for a patient with one or more tumours in the liver were surgery and chemotherapy (the administration of drugs to treat cancer). Some liver tumours, however, are difficult to access surgically, and others could require the surgeon to remove too much of the liver. Moreover, chemotherapy is highly toxic to the liver, and certain tumours do not respond well to it anyway. In some such cases, an interventional radiologist can treat the tumours by disrupting their blood supply, which they need if they are to continue to grow. In this procedure, called radio-embolisation, the radiologist accesses the liver with a fine needle, threaded through one of the patient’s blood vessels. The radiologist then inserts tiny radioactive “seeds” into the blood vessels that supply the tumours. In the days and weeks following the procedure, the radiation emitted from the seeds destroys the vessels and directly kills the tumour cells in the vicinity of the treatment.
How do electrons affect chemical reactions?
In the human body, atoms do not exist as independent entities. Rather, they are constantly reacting with other atoms to form and to break down more complex substances. To fully understand anatomy and physiology you must grasp how atoms participate in such reactions. The key is understanding the behaviour of electrons.
Although electrons do not follow rigid orbits a set distance away from the atom’s nucleus, they do tend to stay within certain regions of space called electron shells. An electron shell is a layer of electrons that encircle the nucleus.
The atoms of the elements found in the human body have from one to five electron shells, and all electron shells hold eight electrons except the first shell, which can only hold two. This configuration of electron shells is the same for all atoms.
The precise number of shells depends on the number of electrons in the atom. Hydrogen and helium have just one and two electrons, respectively. If you take a look at the periodic table of the elements (Figure 3.4), you will notice that hydrogen and helium are placed alone on either sides of the top row; they are the only elements that have just one electron shell (Figure 3.6 (a) and (b)). A second shell is necessary to hold the electrons in all elements larger than hydrogen and helium.
Lithium (Li), whose atomic number is 3, has three electrons. Two of these fill the first electron shell, and the third spills over into a second shell. The second electron shell can accommodate as many as eight electrons. Carbon, with its six electrons, entirely fills its first shell, and half-fills its second (Figure 3.6 (c)). With ten electrons, neon (Ne) entirely fills its two electron shells (Figure 3.6 (d)). Again, a look at the periodic table reveals that all of the elements in the second row, from lithium to neon, have just two electron shells. Atoms with more than ten electrons require more than two shells. These elements occupy the third and subsequent rows of the periodic table. It is useful to remember that the first, second and third electron shells can hold 2, 8 and 8 electrons respectively.
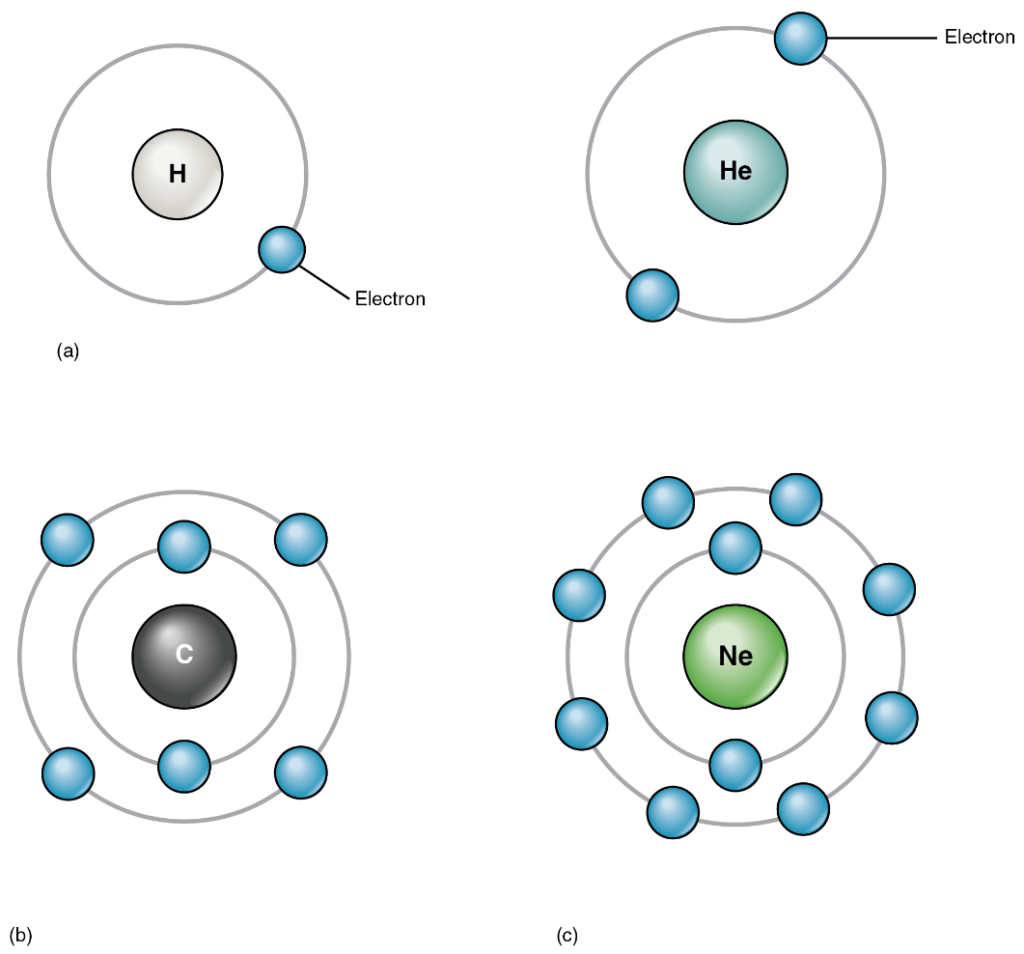
The factor that most strongly governs the tendency of an atom to participate in chemical reactions is the number of electrons in its valence shell. A valence shell is an atom’s outermost electron shell. If the valence shell is full, the atom is stable; meaning its electrons are unlikely to be pulled away from the nucleus by the electrical charge of other atoms. If the valence shell is not full, the atom is reactive; meaning it will tend to react with other atoms in ways that make the valence shell full. Consider hydrogen, with its one electron only half-filling its valence shell. This single electron is likely to be drawn into relationships with the atoms of other elements, so that hydrogen’s single valence shell can be stabilised.
All atoms (except hydrogen and helium with their single electron shells) are most stable when there are exactly eight electrons in their valence shell. For example, oxygen, with six electrons in its valence shell, is likely to react with other atoms in a way that results in the addition of two electrons to oxygen’s valence shell, bringing the number to eight.
In nature, atoms of one element tend to join with atoms of other elements in characteristic ways. For example, carbon commonly fills its valence shell by linking up with four atoms of hydrogen. In so doing, the two elements form the simplest of organic molecules, methane, which also is one of the most abundant and stable carbon-containing compounds on Earth. Another example is water; oxygen needs two electrons to fill its valence shell. It commonly interacts with two atoms of hydrogen, forming H2O. Incidentally, the name “hydrogen” reflects its contribution to water (hydro- = water; -gen = maker). Thus, hydrogen is the water maker.
3.2 | How do elements make compounds?
Atoms separated by a great distance cannot link; rather, they must come close enough for the electrons in their valence shells to interact. But do atoms ever actually touch one another? Most physicists would say no, because the negatively charged electrons in their valence shells repel one another. No force within the human body—or anywhere in the natural world—is strong enough to overcome this electrical repulsion.
Instead, atoms link by forming a chemical bond. A bond is a weak or strong electrical attraction that holds atoms in the same vicinity. The new grouping is typically more stable—less likely to react again—than its component atoms were when they were separate. A more or less stable grouping of two or more atoms held together by chemical bonds is called a molecule. The bonded atoms may be of the same element, as in the case of H2, which is called molecular hydrogen or hydrogen gas. When a molecule is made up of two or more atoms of different elements, it is called a chemical compound. Thus, a unit of water, or H2O, is a compound, as is a single molecule of the gas methane, or CH4.
Three types of chemical bonds are important in human physiology, because they hold together substances that are used by the body for critical aspects of homeostasis, signaling, and energy production, to name just a few important processes. These three types of chemical bonds are ionic bonds, covalent bonds and hydrogen bonds.
What happens when atoms gain or lose electrons?
Recall that an atom typically has the same number of positively charged protons and negatively charged electrons. As long as this situation remains, the atom is electrically neutral. However, when an atom participates in a chemical reaction that results in the donation or acceptance of one or more electrons, the atom will then become positively or negatively charged. This happens frequently for most atoms in order to have a full valence shell, as described previously, because a full valence shell makes the atom stable. This can happen either by gaining electrons to fill a shell that is more than half-full, or by giving away electrons to empty a shell than is less than half-full. Therefore, if an atom gains an electron, the atom acquires a negative electrical charge, and if it loses an electron it acquires a positive electrical charge—whether positive or negative—the atom is known an ion.
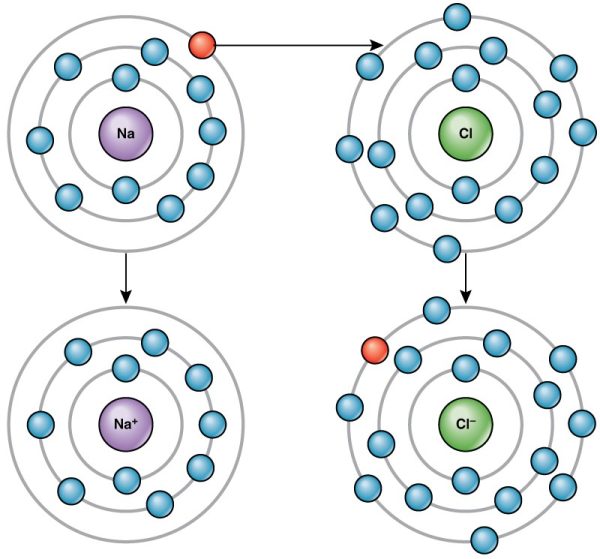
Sodium (Na), for instance, is an important element in all body cells. Its atomic number is 11. It has just one electron in its valence shell. This characteristic makes sodium highly likely to participate in chemical reactions in which it donates one electron. (It is easier for sodium to donate one electron than to gain seven electrons). The loss will cause the positive charge of sodium’s protons to be more influential than the negative charge of the remaining sodium electrons. In other words, the resulting sodium ion will be slightly positive. A sodium ion is written Na+, indicating that it has lost a single electron. A positively charged ion is known as a cation.
Now consider chlorine (Cl), another important element in the body. Its atomic number is 17, and it has seven electrons in its valence shell. Thus, it is highly likely to bond with other atoms in such a way that chlorine accepts one electron (it is easier for chlorine to gain one electron than to donate seven electrons). When it does, its electrons will outnumber its protons by one, and chlorine will have an overall negative charge. The ionised form of chlorine is called chloride, and is written as Cl-. A negatively charged ion is known as an anion.
Atoms that have more than one electron to donate or accept will end up with stronger positive or negative charges. A cation that has donated two electrons has a net charge of +2. Using magnesium (Mg) as an example, this can be written Mg++ or Mg2+. An anion that has accepted two electrons has a net charge of –2. The ionic form of oxygen (O), for example, is typically written O2–.
The opposite charges of cations and anions exert a moderately strong mutual attraction that keeps the atoms in close proximity forming an ionic bond. An ionic bond is an ongoing, close association between ions of opposite charge. The table salt you sprinkle on your food owes its existence to ionic bonding. As shown in Figure 3.7, sodium commonly donates an electron to chlorine, becoming the cation Na+. When chlorine accepts the electron, it becomes the chloride anion, Cl–. With their opposing charges, these two ions strongly attract each other and become the compound Sodium Chloride (NaCl).
You could say that the relationship between sodium and chlorine is a love story! A love story that is disrupted by water. Water is an essential component of life because it is able to break the ionic bonds in salts to free the ions. In fact, in biological fluids, most individual atoms exist as ions.
Therefore, when sodium chloride dissolves in water, the ionic bond between sodium and chloride ions is disrupted; the properties of water which allow this disruption will be discussed in the section on polar covalent bonds later in the chapter.
What happens when atoms share electrons?
Unlike ionic bonds formed by the attraction between a cation’s positive charge and an anion’s negative charge, molecules formed by a covalent bond share electrons in a mutually stabilising relationship. Like next-door neighbours whose kids hang out first at one home and then at the other, the atoms do not lose or gain electrons permanently. Instead, the electrons move back and forth between the elements. Because of the close sharing of pairs of electrons (one electron from each of two atoms), a covalent bond is stronger than an ionic bond. There are two types of covalent bonds, non-polar and polar.
Non-polar covalent bonds – sharing equally
Figure 3.8 shows several common types of covalent bonds. Notice that the two covalently bonded atoms typically share just one or two electron pairs, though larger shares are possible. The important concept to take from this is that in covalent bonds, electrons in the outermost valence shell are shared to fill the valence shells of both atoms, ultimately stabilising both of the atoms involved. In a single covalent bond, a single electron is shared between two atoms, while in a double covalent bond, two pairs of electrons are shared between two atoms. There are even triple covalent bonds, where three atoms are shared.
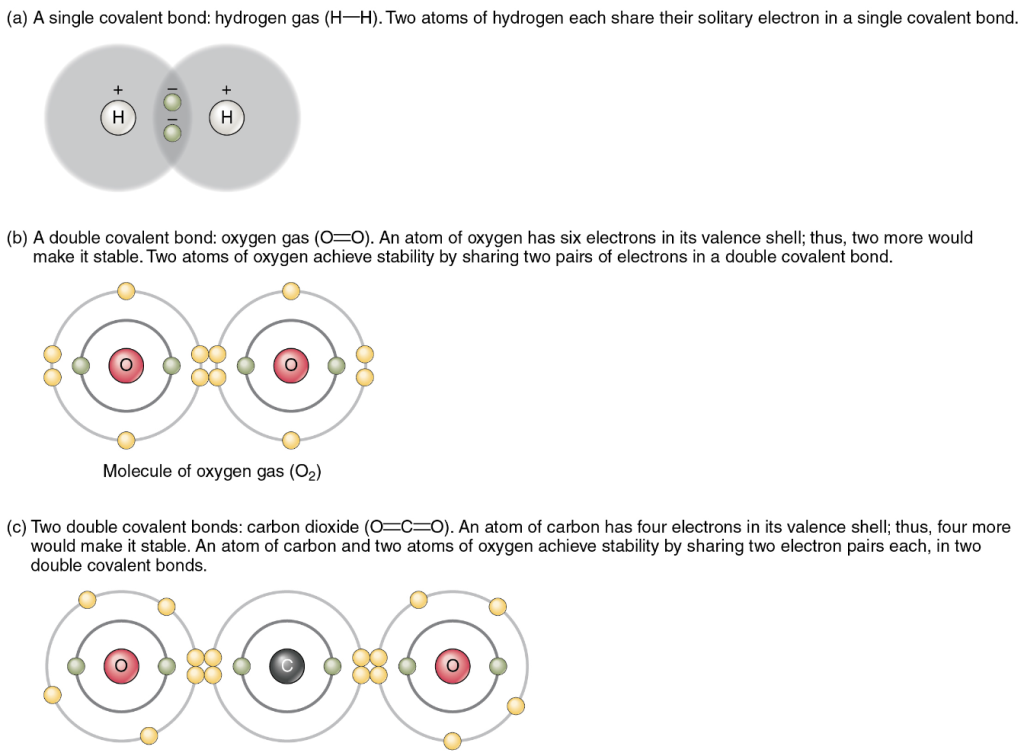
You can see that the covalent bonds shown in Figure 3.8 are balanced, that is, the sharing of the negative electrons is relatively equal, as is the electrical pull of the positive protons in the nucleus of the atoms involved. This is why covalently bonded molecules that are electrically balanced in this way are described as nonpolar; that is, no region of the molecule is either more positive or more negative than any other.
Polar covalent bonds – unequal sharing
Groups of legislators with completely opposite views on a particular issue are often described as polarised by news writers. In chemistry, a polar molecule is a molecule that contains regions that have opposite electrical charges. Polar molecules occur when atoms share electrons unequally in polar covalent bonds.
When two hydrogen atoms each share their single electron with oxygen, covalent bonds are formed, resulting in a molecule of water, H2O. Water is the most familiar example of a polar molecule (Figure 3.9). The molecule has three parts: one atom of oxygen and two hydrogen atoms. The oxygen nucleus contains eight protons, and the two hydrogen nuclei each contain only one proton. Because every proton exerts an identical positive charge, a nucleus that contains eight protons (oxygen) exerts a charge eight times greater than a nucleus that contains one proton (hydrogen). This means that the negatively charged electrons present in the water molecule are more strongly attracted to the oxygen nucleus than to the hydrogen nuclei. Each hydrogen atom’s single negative electron therefore migrates toward the oxygen atom, making the oxygen end of their bond slightly more negative than the hydrogen end of their bond.
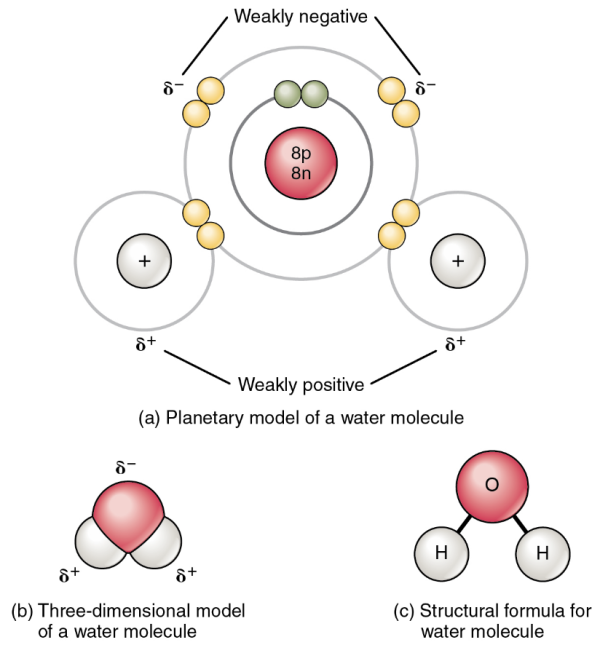
What is true for the bonds is true for the water molecule as a whole, that is, the oxygen region has a slightly negative charge and the regions of the hydrogen atoms have a slightly positive charge. These charges are often referred to as partial charges because the strength of the charge is less than one full electron, as would occur in an ionic bond. As shown in Figure 3.9, regions of weak polarity are indicated with the Greek letter delta (δ) and a plus (+) or minus (–) sign.
Even though a single water molecule is unimaginably tiny, it has mass, and the opposing electrical charges on the molecule pull that mass in such a way that it creates a shape somewhat like a triangular tent (see Figure 3.9b). This dipole, with the positive charges at one end formed by the hydrogen atoms at the bottom of the tent and the negative charge at the opposite end (the oxygen atom at the top of the tent) makes the charged regions highly likely to interact with charged regions of other polar molecules. For human physiology, the resulting bond is one of the most important formed by water—the hydrogen bond.
3.3 | Chemical reactions
All chemical reactions begin with a reactant, the general term for the one or more substances that enter into the reaction. Sodium and chloride ions, for example, are the reactants in the production of table salt. The one or more substances produced by a chemical reaction are called the product.
In chemical reactions, the components of the reactants—the elements involved and the number of atoms of each—are all present in the product(s). Similarly, there is nothing present in the products that is not present in the reactants. This is because chemical reactions are governed by the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Just as you can express mathematical calculations in equations such as 2 + 7 = 9, you can use chemical equations to show how reactants become products. As in maths, chemical equations proceed from left to right, but instead of an equal sign, they employ an arrow or arrows indicating the direction in which the chemical reaction proceeds. For example, the chemical reaction in which one atom of nitrogen and three atoms of hydrogen produce ammonia (NH3) would be written as N + 3H → NH3. Correspondingly, the breakdown of ammonia into its components would be written as NH3 → N + 3H.
Notice that, in the first example, a nitrogen (N) atom and three hydrogen (H) atoms bond to form the compound, NH3. This reaction requires energy, which is then stored within the compound’s bonds. Such reactions are referred to as synthesis reactions. A synthesis reaction is a chemical reaction that results in the synthesis (joining) of components that were formerly separate (Figure 3.10a). Again, nitrogen and hydrogen are reactants in a synthesis reaction that yields ammonia as the product. The general equation for a synthesis reaction is A + B → AB.
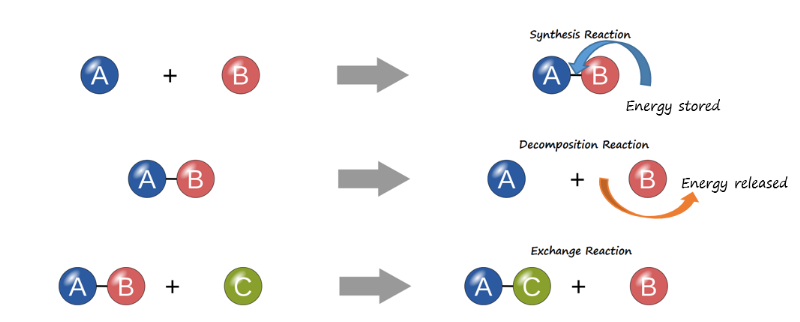
In the second example, ammonia is catabolized into its smaller components, and the potential energy that had been stored in its bonds is released. Such reactions are referred to as decomposition reactions. A decomposition reaction is a chemical reaction that breaks down or “de-composes” something larger into its constituent parts (see Figure 3.10b). The general equation for a decomposition reaction is: AB → A + B.
An exchange reaction is a chemical reaction in which both synthesis and decomposition occur, chemical bonds are both formed and broken, and chemical energy is absorbed, stored, and released (see Figure 3.10c). The simplest form of an exchange reaction might be: A + BC → AB + C. Notice that, to produce these products, B and C had to break apart in a decomposition reaction, whereas A and B had to bond in a synthesis reaction. A more complex exchange reaction might be: AB + CD → AC + BD. Another example might be: AB + CD → AD + BC.
In theory, any chemical reaction can proceed in either direction under the right conditions. Reactants may synthesise into a product that is later decomposed. Reversibility is also a quality of exchange reactions. For instance, A + BC → AB + C could then reverse to AB + C → A + BC. This reversibility of a chemical reaction is indicated with a double arrow: A + BC ⇄ AB + C. Still, in the human body, many chemical reactions do proceed in a predictable direction, either one way or the other. You can think of this more predictable path as the path of least resistance because, typically, the alternate direction requires more energy.
In theory, any chemical reaction can proceed in either direction under the right conditions. Reactants may synthesise into a product that is later decomposed. Reversibility is also a quality of exchange reactions. For instance, A + BC → AB + C could then reverse to AB + C → A + BC. This reversibility of a chemical reaction is indicated with a double arrow: A + BC ⇄ AB + C. Still, in the human body, many chemical reactions do proceed in a predictable direction, either one way or the other. You can think of this more predictable path as the path of least resistance because, typically, the alternate direction requires more energy.
Crossword
anion an atom which carries a negative charge
atomic mass the sum of the protons and neutrons in an atom
atomic number the number of protons in an element
cation an atom which carries a positive charge
chemical bond any rearrangement of electrons in two atoms generates a force, causing the atoms to be bound together
compound is formed when two elements are chemically bonded together
covalent bond is a chemical link between two atoms where the electron pairs are shared between them
electron sub-atomic particle that orbits around the nucleus of an atom. It carries a charge of -1
electron shell group of orbitals followed by electrons around the nucleus of an atom
element the simplest substances that cannot be broken down by chemical means
hydrogen bond electrostatic attraction between a hydrogen ion in one molecule and an electronegative atom in another molecule
ion an atom that carries a positive or negative charge
ionic bond a chemical bond between two ions with opposite charges
molecule forms when 2 or more atoms form chemical bonds; it does not matter if the atoms are the same or different from one another
nucleus positively charged centre of atoms. It is made up of protons and neutrons, except in the case of the hydrogen atom which has one proton and no neutron
neutron subatomic particle found in the nucleus an atom. It has the same mass as a proton but has no charge
product a substance produced in a chemical reaction
proton subatomic particle found in the nucleus of an atom. It has the same mass as a neutron but carries a charge of +1
polar molecule has a partial positive charge in one part of the molecule and complementary negative charge in another part
reactant a chemical substance that is present at the start of a chemical reaction
valence electron an electron of an atom, located in the outermost shell, that can be transferred to or shared with another atom
valence shell the outermost shell of an atom containing the valence electrons
valency is the number of electrons an atom will accept, donate or share to form a chemical bond
Review questions
Chapter attribution
Content adapted from:
Anatomy and Physiology 2e (2022), by J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble and Peter DeSaix, is published by OpenStax https://openstax.org/details/books/anatomy-and-physiology-2e, and used under a CC BY licence.
Concepts of Biology (2013), by Samantha Fowler, Rebecca Roush and James Wise, is published by OpenStax https://openstax.org/details/books/concepts-biology, and used under a CC BY licence.
Microbiology (2016), by Nina Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister and Brian M. Forster, is published by OpenStax https://openstax.org/details/books/microbiology, and used under a CC BY licence.
Media Attributions
- Figure 3.1 Carbon atom © Available under a Pixabay licence
- Figure 3.2 Elements of the human body © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix is licensed under a CC BY (Attribution) license
- Figure 3.3 Planetary model of an atom © Samantha Fowler, Rebecca Roush & James Wise is licensed under a CC BY (Attribution) license
- Figure 3.4 Periodic Table of Elements © Connie Rye, Robert Wise, Vladimir Jurukovski, Jean DeSaix, Jung Choi & Yael Avissar is licensed under a CC BY (Attribution) license
- Figure 3.5 Isotopes of Hydrogen © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix is licensed under a CC BY (Attribution) license
- Figure 3.6 Electron shells © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix is licensed under a CC BY (Attribution) license
- Figure 3.7 Ionic bonding © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix is licensed under a CC BY (Attribution) license
- Figure 3.8 Covalent bonding © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix is licensed under a CC BY (Attribution) license
- Figure 3.9 Polar covalent bonding © J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble & Peter DeSaix is licensed under a CC BY (Attribution) license
- 3.10 Fundamental chemical reactions © Daniele Pugliesi adapted by Vicki Dunk is licensed under a CC BY-SA (Attribution ShareAlike) license

