9.4 Oxygen Therapy, Hyperbarics and the Gas Laws
Learning Outcomes
Be able to:
- Describe the significance of the different gas laws to respiratory physiology.
- Explain the benefit of oxygen therapy by applying your knowledge of Dalton’s laws
- Explain the use of hyperbaric oxygen therapy by applying your knowledge of Henry’s laws
- Describe how inspiration occurs in negative pressure breathing animals such as humans by applying your knowledge of Boyle’s law.
Introduction
In this chapter, we will explore the relationship between the gas laws: Dalton’s Law, Henry’s Law and Boyle’s Law to oxygen therapy and the oxygenation of blood.
Dalton’s Law
According to Dalton’s law, each gas in a mixture of gases exerts its own pressure as if no other gases were present. The pressure of a specific gas in a mixture is called its partial pressure. In the example of arterial blood gases below, we see the partial pressure of the different gases reported in mm/Hg.
- pH7.25 (Normal range 7.35 – 7.45)
- pCO2 74 mmHg (Normal range 35 – 45 mm/Hg)
- pO2 17 mmHg (Normal range 80 – 100 mm/Hg)
- HCO3 33 mmol/L (Normal range 22-26 mmol/L)
If we consider air – a mixture of a number of gases. By volume, dry air contains
- 78.09%nitrogen,
- 20.95%oxygen,
- 0.93%argon,
- 0.04%carbon dioxide,
- small amounts of other gases.
- Air also contains a variable amount of water vapor (on average between 0.4-1% but varies considerably due to atmospheric conditions).
As shown in the diagram below, the total pressure of the mixture is calculated by adding all the partial pressures.

Image taken from Dalton’s law of partial pressure Khan Academy presentation available at https://www.khanacademy.org/science/chemistry/gases-and-kinetic-molecular-theory/ideal-gas-laws/a/daltons-law-of-partial-pressure Image adapted from OpenStax, CC BY 3.0.
- For example, we can work out the partial pressure of oxygen in the atmosphere at sea level (1ATM)
- 20.95%x 760mm/Hg = PaO2 159.22 mm/Hg.
📺 Watch: If you want to hear this another way – Professor Dave provides a useful explanation of Dalton’s law and partial pressures (albeit with corny background music). This is available below and goes for about 6:36 minutes.
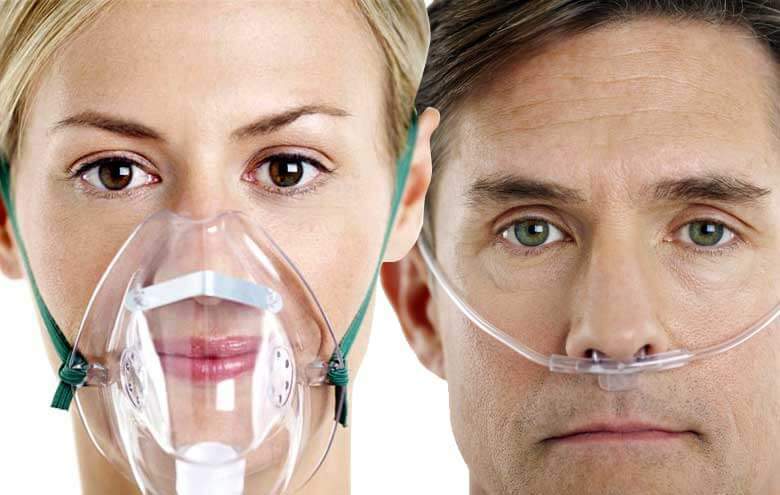
This same law applies to the blood –there is a partial pressure of oxygen or CO2 or any other gas in the blood. So, what is the importance of this? Well, if we increase the fraction of inspired oxygen using oxygen therapy, we can increase the alveolar partial pressure as well as the partial pressure of oxygen in the blood. This will increase the delivery of oxygen to tissues. We can increase the fraction of inspired oxygen FiO2 using oxygen masks or nasal prongs.
Note: The lecture recording at the end of this chapter will describe the application of Dalton’s law in more detail in relation to oxygen therapy.
Henry’s Law
Henry’s law states that the quantity of gas that will dissolve in a liquid is proportional to the partial pressure of the gas and the solubility.
- CO2 is very soluble in water (or blood) – about 24 times more than O2
- O2 is soluble in water (or blood) – more than nitrogen
- N2 is less soluble in water or blood (less than half as soluble as O2)
So Henry’s law tells us as the partial pressure of a gas increases – the solubility increases. Lets look at some examples of the partial pressure of oxygen:
- Normal air at sea level (1 ATM or 760mm/Hg)
- 760mm/Hg x 21% = PaO2 152 mm/Hg.
- Normal air at 2 atmospheres (1520 mm/Hg) in a hyperbaric dive chamber
- 1520mm/Hg x 21% = 319.2 mm/Hg
- Normal air at 3000m elevation with a barometric pressure of 522mm/Hg
- 522mm/Hg x 21% = 109.62 mm/Hg
Note the differences in the partial pressure of oxygen. As pressure increases, the partial pressure of the gas increases. The opposite happens at lower pressures – the partial pressure decreases. Henry’s Law tells as as the partial pressure of the gas increases, the solubility of the gas in solution also increases. This is useful if we want to increase the solubility of oxygen in the blood. We can increase the FiO2 (fraction of inspired oxygen) but also the solubility of oxygen by increasing the pressure. We do this in hyperbaric chambers. Hyperbaric chambers are better know for their use in the management of decompression illness (or the ‘bends’) but they have other uses.
Boyle’s law states the pressure of gas in a closed container is inversely proportional to the volume of the container. So if we have a closed container with a volume of X and there is 1ATM of gas in there and we reduce the volume of the container to half the original volume – the pressure will inversely increase – that is the pressure will double.
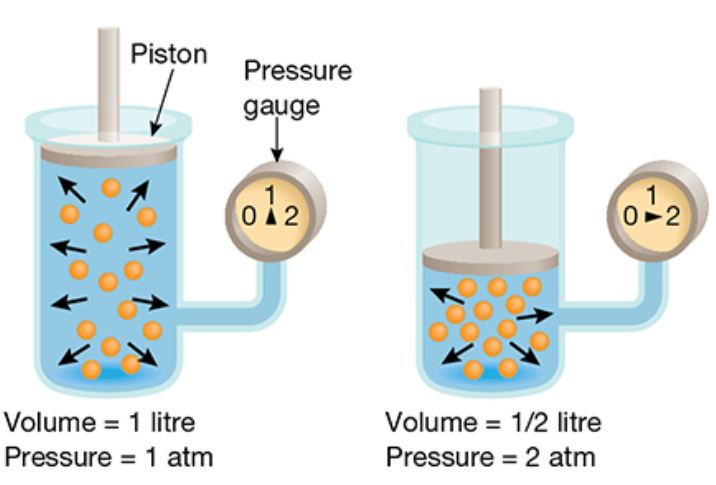
Image sourced from Tortora, GJ., Derrickson, B., Burkett, B., Peoples, G., Dye, D., Cooke, J., et al. Principles of anatomy and physiology. Second Asia-Pacific ed. Queensland, Australia: John Wiley & Sons; 2019.
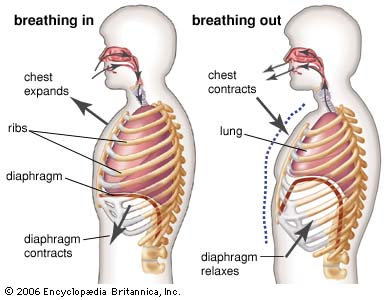
So what? Well, it is Boyle’s law that explains to us how we create the inward flow of air into our lungs during respiration. When we inhale, we move the diaphragm down and the ribs in the rib cage pivot our just like the handle on a bucket. This causes the expansion of the chest. Lets say the volume of the space in the lungs doubles – according to Boyle’s law, the expansion of the chest lowers the pressure in the lungs (increased volume = decreased pressure) and air moves in from the higher pressure space in the atmosphere – this is negative pressure breathing and explains why we can move air in to our lungs.
The elastic recoil of the lungs and relaxation of the respiratory muscles decreases the volume of the lungs – pressure in the lungs increase and air is pushed out.
OK, that’s about all you need to know about oxygen therapy, hyperbarics and gas laws!