6.1.2 Acute Complications of Diabetes (DKA, HHS and Hypoglycaemia)
Acute Complications of Diabetes
Learning Outcomes
Be able to:
- Describe the condition of DKA and the change in insulin and counter regulatory hormones in DKA.
- Relate the symptoms of DKA to patient presentation and relate the pathophysiology of DKA to diabetes and the triad of presenting symptoms.
- Describe the basic approach to the emergency management of DKA.
- Describe the condition of HHS and relate the pathophysiology of HHS to diabetes and the triad of presenting symptoms.
- Describe the fluid changes associated with HHS and impact on the circulatory system.
- Describe the basic approach to the emergency management of HHS including fluid resuscitation, BGL correction, hypokalaemia, arterial/venous thromboembolism risk reduction.
- Justify the risks of rapid correction of BGL on cardiovascular system.
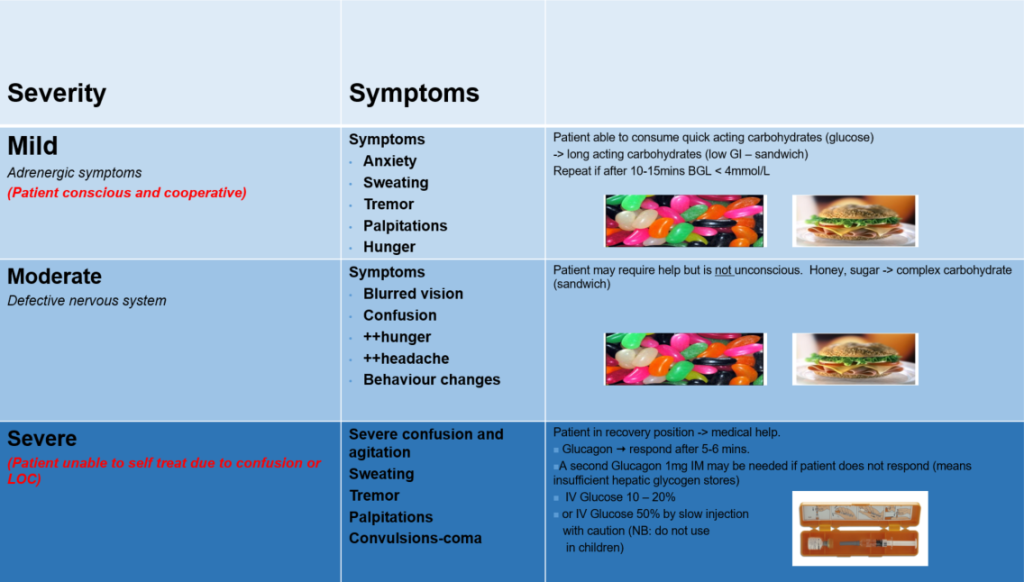
- Recognise the signs and symptoms of hypoglycaemia and identify the risk factors for hypoglycaemia.
- Be able to differentiate the difference between mild and moderate hypoglycaemia and severe hypoglycaemia.
- Describe the treatment of mild and moderate and severe hypoglycaemia
- Be able to use a GlucaGen Hypokit and know the dose for children under the age of 12 and children and adults > 12 years of age.
Diabetic Ketoacidosis (DKA)
Introduction to DKA
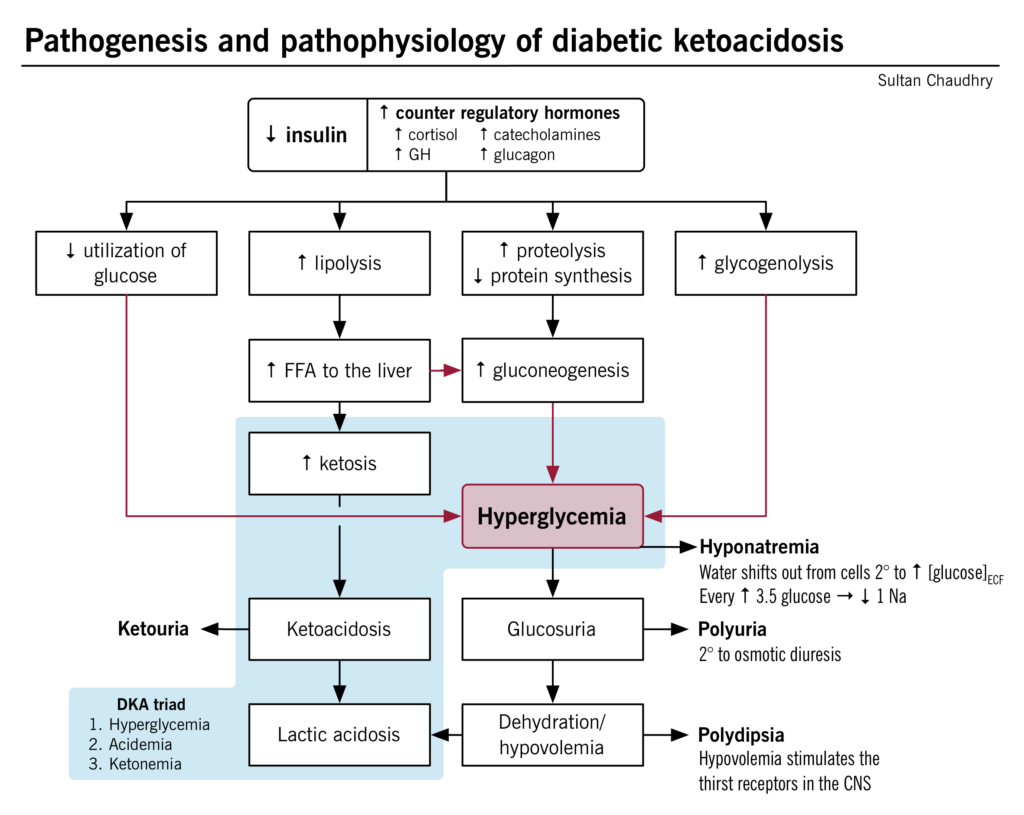
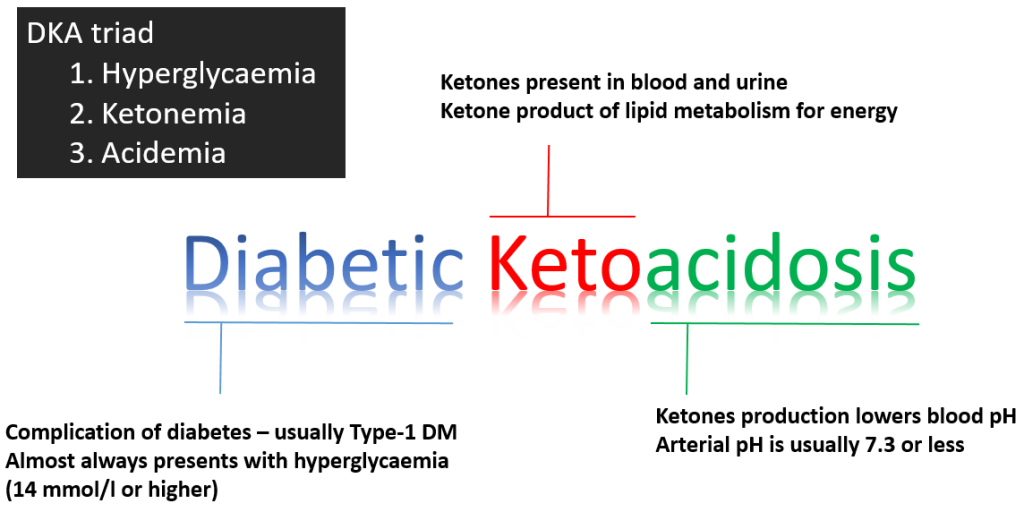
Diabetic ketoacidosis (DKA) is a medical emergency that should be managed under specialist supervision. DKA is primarily a disease of Type 1 diabetes mellitus and presents as a triad of symptoms:
- Hyperglycaemia secondary to an absolute insulin deficiency. This normally presents as a Blood Glucose Level (BGL) above 14 mmol/L.
- Ketonemia resulting from unopposed lipolysis (unopposed because of the absolute insulin deficiency) and the conversion of the free fatty acids to ketones by the liver (ketones are a source of energy utilised by peripheral tissues and not reliant upon insulin for transportation into the cell). Ketones are present in both the blood, urine and can be detected on the breath (as ketones are volatile).
- Acidemia resulting from the conversion (by the liver) of the free fatty acids to ketones which are acidic and reduce blood pH. In DKA, blood pH is normally ≤ 7.3. (normal range is 7.35-7.45)
Hyperosmotic Hyperglycaemia State (HHS)
Introduction to Hyperosmotic Hyperglycaemia State (HHS)
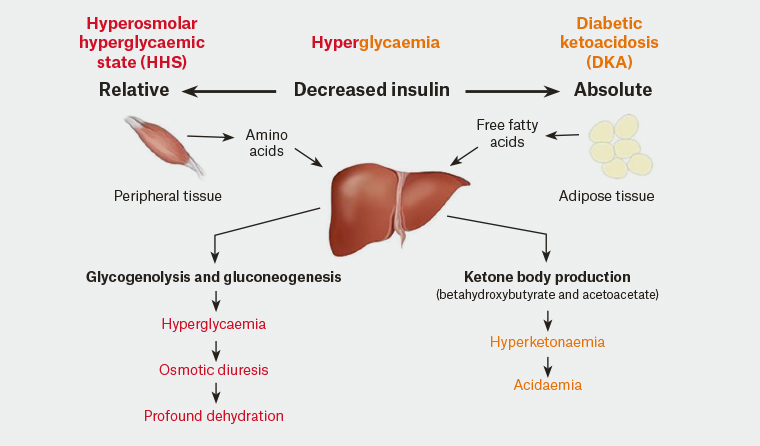
Hyperosmolar Hyperglycaemic State (HHS) is a severe complication of (typically) type 2 diabetes. The disease is characterized by significant dehydration secondary to significant hyperglycaemia. The hyperglycaemia is typically prolonged and much higher than is seen in Diabetic Ketoacidosis (DKA). The typical blood glucose levels (BGL) is often greater than 30 mmol/L. The key differences between DKA and HHS are
- There is often no insulin production in DKA but there is some residual pancreatic insulin activity in HHS meaning conversion of free fatty acids to ketones by the liver does not occur.
- There is no ketone (or very minimal ketone) production meaning ketosis and acidosis is not a major feature of HHS.
- Dehydration is much more severe due to the hyperosmolar condition caused by the super-high BGL.
- Hyperosmolarity occurs due to the cycle of increased diuresis —> dehydration —>increased osmolality —> increased diuresis
- The dehydration caused in HHS is much more significant than in DKA – leading to circulatory collapse
Given the pathophysiology of HHS and the effect on the cardiovascular system, without treatment, circulatory collapse and coma can occur. This makes HHS arguably more serious than DKA and this is evidenced by the higher mortality rate associated with HHS. Symptoms of HHS include:
- Hyperglycaemia
- Glucosuria
- Hypokalaemia
- Polyuria
- Neurological changes – confusion, stupor and coma
- Severe dehydration leading to circulatory collapse (shock) and coma.
Cause of HHS
HHS is caused by a relative lack of insulin production coupled with increases in circulating levels of the counter regulatory hormones glucagon, adrenaline, cortisol and growth hormone. The lack of insulin-induced skeletal muscle uptake of glucose causes amino acid release which stimulates counter regulatory hormone release and increased production of glucose by the liver through glycogenolysis and gluconeogenesis. This effectively raises the blood glucose levels causing hyperglycaemia. The hyperglycaemia causes osmotic diuresis and profound dehydration.
Fluid shifts caused by the osmotic diuresis and severe hyperglycaemia
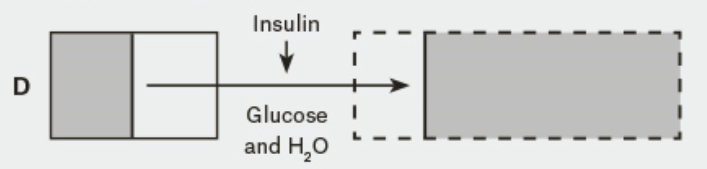
Under normal osmotic and glycaemic concentrations (see Figure A in the diagram below), the intracellular fluid (ICF) and extracellular fluid (ECF) have balanced and normal concentrations of glucose and therefore osmotic concentrations (all things being equal). This is because in the normal non-diabetic state, blood glucose concentrations between the ECF and ICF remain balanced (because insulin allows the uptake of glucose from the blood into the cells where it is used).
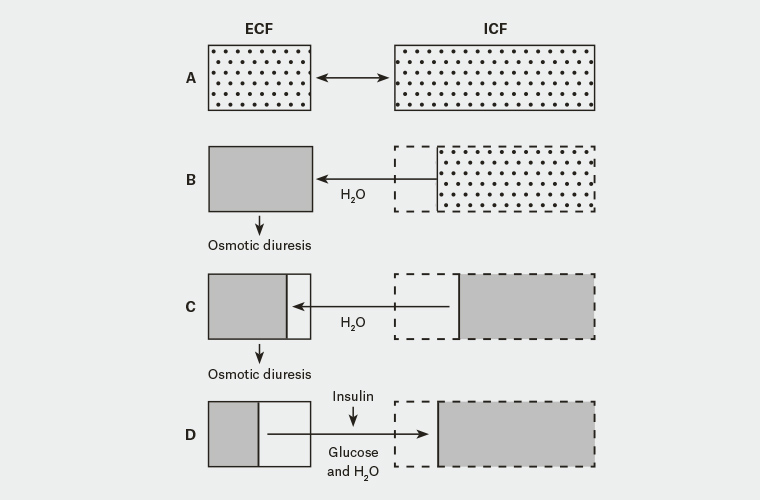
In type 2 diabetes where there is a relative reduction in the availability of insulin, the blood concentration of glucose (in the ECF) increases (see figure B in the diagram above). To balance the osmolarity difference between the ECF and ICF, water shifts from the ICF to the ECF. Given the still relatively high (and still increasing blood glucose levels due to the release of the counter regulatory hormones) the ECF is still high in glucose and hyperosmolar.
- Because of the hyperosmolar state, osmotic diuresis ensues and significant amounts of urine (and some glucose) are lost in the urine. This further increases the osmolarity of the ECF (more fluid is lost and more glucose is produced). The ICF loses water to the ECF in an attempt to reduce the osmolar gradient between the ICF and the ECF further depleting the hydration of the ICF. Continued osmotic diuresis causes more water loss via the kidneys and when this gets critical, hypovolaemia, shock, circulatory collapse and coma can occur (see Figure C of the diagram above).
Management of HHS – fluid resuscitation
HHS is initially managed with fluid resuscitation to correct volume deficit and electrolyte balance. 0.9% sodium chloride solution is used. This has the effect of also effectively decreasing the glucose concentration and therefore the hyperosmolar state as the concentration of glucose is reduced and at the same time correcting the fluid deficit caused by the hyperglycaemia. Given the significant excretion of glucose, significant other electrolyte losses are often experienced including magnesium, phosphate and potassium.
Potassium is the most troublesome typically and so potassium chloride may be added to the fluid resuscitation plan in subsequent bags to elevate this crucial electrolyte. In addition to the slow correction of BGL and the treatment of the cause of the patients HHS (this requires investigation), the patient is also at risk of developing arterial and venous thrombosis. The short-term immediate risk of arterial and venous thrombosis is managed using low molecular weight heparin or unfractionated heparin.
Management of HHS – correction of blood glucose levels
The infusion of 0.9% sodium chloride will have the effect of lowering the BGL and osmolality and should be allowed to reduce slowly aiming for a target of between 10 to 15 mmol/L. If blood glucose levels stop falling after initial fluid replacement, an intravenous insulin infusion of a short acting insulin such as Actrapid can be given. Caution should be taken in the early administration of insulin in HHS as circulatory collapse can occur.
Caution: Rapid correction of blood glucose levels can lead to circulatory collapse
Early administration of insulin in HHS risks circulatory collapse. This occurs when excess glucose in transported from the ECF to the ICF. This shift in glucose into the cells effectively decreases ECF osmolality and increases ICF osmolality to such an extent it encourages a significant shift of fluid from out of the ECF further reducing circulating volume (see figure D of diagram).
That coupled with ongoing diuresis and an already low ECF volume cases circulatory collapse.